|
|
|
Indian Pediatr 2009;46: 957-962 |
 |
Cost-effectiveness of Supplementary
Immunization for Measles in India |
|
Mayank Dabral
From the Department of Health Policy and Management,
Mailman School of Public Health, Columbia University,
New York, NY USA.
Correspondence to: Dr Mayank Dabral, 276, 1st Street, Apt
# 3J, Mineola, Long Island, New York, NY 11501, USA.
E-mail:
[email protected]
Manuscript received: October 10, 2008;
Initial review: October 30, 2008;
Accepted: March 6, 2009.
Published online: 2009 May 20.
PII: S097475590800597-1 |
|
Abstract
Objective: This study aims to estimate the
incremental cost effectiveness of a supplementary immunization activity
(SIA) for measles in a district of India with measles vaccine coverage
equivalent to the National average.
Design: A state transition model is used to
estimate the effect of routine vaccination with measles vaccine as well
as with measles vaccine during the SIA. The model follows each
sub-cohort in the target population at respective age (1-5 years) to
five years of age, using age specific incidence rate and vaccination
rate to determine the number of cases of measles. Using age specific
incidence rates and complication rates for measles; deaths and
disability adjusted life year (DALY) averted is estimated.
Results: Using base-case assumptions, an
estimated 65479 cases of measles and 1637 deaths due to measles will be
prevented in a span of four years from a single supplementary
immunization activity in a pediatric population (1-5 years of age) of
size 839,473. The cost per measles vaccine dose delivered is INR 30.
Using base case analysis the cost to avert a death is INR 15381 and the
cost per disability adjusted life year (DALY) averted is INR 430.
Conclusions: Supplementary immunization activity
for measles is cost-effective. However, this cannot be considered
superior to a second dose of measles in routine immunization.
Key Words: Cost-effectiveness, India, Measles, Vaccine,
Supplementary immunization.
|
|
D
ue to the availability of an
efficient vaccine which provides long term immunity, and the fact that
measles is generally limited to the pediatric population, measles is
considered as the next disease targeted for eradication. The World Health
Organization and United Nations Children Fund have come up with a Measles
Vaccine Initiative focused towards controlling and eradicating measles in
the developing countries. In India, a single dose of measles vaccine is
offered to every child at the age of nine months under the National
Immunization Program. Several developed countries have national
immunization programs offering upto three doses of the measles vaccine.
The current strategy for measles mortality reduction in India by two
thirds by 2010, focuses on a second opportunity for measles immunization
through routine immunization in states where routine measles coverage
exceeds 90% and local resources are available to sustain the strategy(1).
However, this does not address the question of measles eradication
strategy for almost 38% of districts of India with measles coverage less
than fifty percent(2).
One of the key activities identified to improve routine
immunization coverage rates is Supplementary Immunization activity (SIA)
in low coverage states. In the past, countries in the American
subcontinent have adopted SIA campaigns which have led to improvements in
routine immunization services and surveillance system(3). In India, this
strategy has been used successfully for pulse immunization for polio,
outbreak control, and crisis management in low coverage areas to rapidly
achieve high coverage. United Nations Children Fund (UNICEF), in
collaboration with the Ministry of Health and Family Welfare, Government
of India has in the past conducted such activities as part of the urban
measles control strategy.
This paper presents a cost effectiveness analysis from
the provider’s perspective. It provides decision makers with evidence to
make a case for conducting supplementary immunization activity for measles
in low coverage districts in India.
Methods
The state of health of the theoretical pediatric cohort
was modeled using TreeAge Pro and Microsoft Excel software. A Markov model
was constructed to estimate the health outcomes in two hypothetical
cohorts of children in India. One cohort received second dose of measles
vaccine through SIA, whereas the other did not; the cohorts were similar
in all other respects.
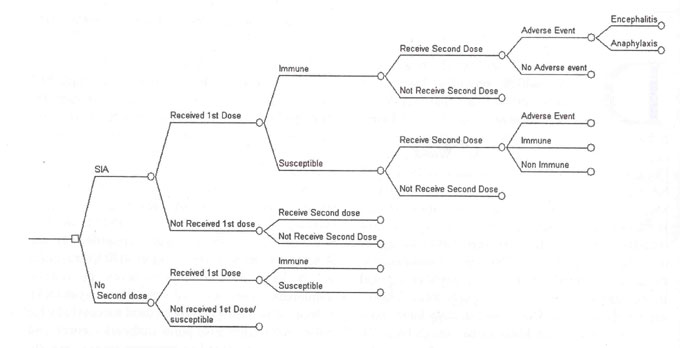
A simplified decision tree diagram is presented in
Fig. 1. In the model it was assumed that everyone in the age
group of 1-5 years is eligible for a dose of measles vaccine during SIA;
including children with previous history of measles infection or
immunization. The main cohort has been divided into four
sub-cohorts according to age groups (1-2yrs, 2-3yrs, 3-4yrs, and 4-5yrs)
to determine the actual number of children who would be susceptible to
measles according to the age specific transmission rates, measles vaccine
coverage and efficacy rates. Children who may have developed immunity
following the first dose of vaccine or an episode of measles were not
included in the susceptible group. The study of complication rates has
been limited to five years of age, beyond which the complication rates due
to measles are not well documented. Consequences of disease are considered
over the lifetime of individuals in the cohort.
 |
|
Fig.1 Simplified decision tree:
proximal branches. |
Epidemiologic data
Probability estimates were obtained from articles
in peer reviewed journals. Studies were identified through a Medline
search and whenever possible the data were collected from published Indian
scientific literature. Age specific transmission rates were obtained from
pre-vaccine era literature(6).
A cohort of 8,39,473 as reported by the WHO
Office for the National Polio Surveillance Program for an Indian
district(7)was used to determine total costs of the campaign, disability
adjusted life years (DALYs) and cases averted comparing the measles
supplementary immunization activity with the status quo of the national
pulse polio campaign activity. For routine immunization activity, baseline
coverage rate of 58% with variation between 30-90% was used, based on the
national average for measles vaccine coverage and state level coverage
rates(8). Baseline coverage rate of 75 percent with variation between
69-96% was used for the supplementary immunization activity, based on the
level achieved in past mass measles vaccination campaign(1). Case fatality
in measles was calculated using median case fatality ratio of 2.5 percent
(C.I. 0.2 to 3.7%) from prospective community based studies in India(9).
Age specific death rate of 0.4% per annum was used to account for a
dynamic population (Table I).
Table I
Incidence Data on Measles Related Sequelae
|
Data |
Incidence value |
Range used in |
Average Duration (age |
|
|
|
sensitivity analysis |
weighted and discounted |
|
|
|
|
at 3%) (assumed) |
| Case fatality ratio |
2.5 % |
0.2 - 3.7%(9) |
| Diarrhea |
30% |
20 - 72%(18 ) |
1 week |
| Pneumonia |
20% |
10 - 30 %( 19) |
1 week |
Malnutrition
(kwashiorkor/marasmus) |
3.5% |
3 - 4 % (20 )
|
30 days |
| Keratomalacia |
0.1% |
0.05 - 0.2(21) |
34.8 years |
| Otitis media |
5% |
5-15 %(22) |
2years |
|
Encephalitis |
1/1,000,000 doses; |
|
34.8 years |
|
|
1-2 cases/1000 |
|
|
|
|
cases(20) |
|
|
| Subacute sclerosing pan |
8.5 cases/million |
|
36.7 years |
| encephalitis |
cases(12) |
|
|
An earlier study shows that adverse reaction to the
measles vaccine is more likely to be related to toxic shock syndrome due
to the use of unsterile syringes and needles, and perhaps the use of
reconstituted vaccines beyond their specified time for administration
resulting in contamination(10). Side effects specifically attributable to
the measles vaccine are fever (5-15 %), rash (5%), encephalitis
(1/1000,000 doses), and anaphylaxis (1/1000,000 doses)(11,12).
Vaccine effectiveness determined by case reference
method has been found to be widely varying from as low as 46% to as high
as 100%(15), and a median value of 85 % by seropositivity methods(16).
Effectiveness for this evaluation was determined at 85% with variation
between 45-100%, used in sensitivity analyses.
Cost data
The measles vaccine costs Rs. 41.71 per vial(1).
Additional dose wastage, transport cost, handling charges and use of
syringes were all factored into the vaccine cost at levels prescribed
under the National Policy for Universal Immunization Program(1).
Additional costs including time cost, travel cost, surveillance cost,
campaign cost and cold chain maintenance cost were taken as equivalent to
that in the National pulse polio campaign.
Cost of injection waste disposal plan was assumed to be
1/3rd of procuring syringes and needles. The total cost of the SIA
campaign came to INR 2,51,77,095. The cost per measles vaccine dose
delivered is INR 30.
Disability Adjusted Life Years
In this study, a 3% discount rate was applied to the
calculation of DALYs, and standardized life expectancy according to age
has been used as in the Global Burden of Disease Study and the Disease
Control Priorities Project(18). Disability weights were apportioned
according to the Global Burden of Disease Study. Using base case scenario,
58,638 DALYs will be averted over a span of four years.
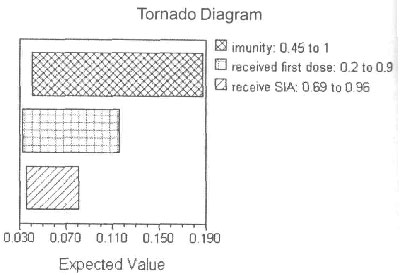
Sensitivity analysis
One-way, two-way and three-way sensitivity analyses
were conducted on variables associated with the greatest degree of
uncertainty including the probability of developing immunity, probability
of developing measles and vaccine coverage rates through routine
immunization and through supplementary immunization. The overall result
was still cost effective, assuming a willingness-to-pay of US $950 per
DALY averted. The overall cases averted were most sensitive to changes in
probability of developing immunity following measles vaccination, followed
by vaccine coverage rates through routine immunization activity (RIA), and
followed by the SIA coverage rates (Fig.2).
 |
|
Fig.2 Sensitivity analyses for the
variables with maximum variability (expected value here is the
probability of developing measles for a single person). |
Results
If no supplementary immunization activity is conducted,
1,39,982 children in this cohort are expected to have an episode of
measles infection in the next 4 years. 3500 deaths would result due to
measles in this cohort. The burden of disease and its sequelae would be
1,25,349 DALYs. A supplementary immunization activity by reducing the
number of susceptible in the population would avert 65479 cases and 1637
deaths, and lower the disease burden by 58638 DALYs. The cost of
implementing the supplementary immunization activity is approximately INR
25.18 million (Year 2008). The incremental cost-effectiveness ratio (ICER)
would be INR 430 per DALY averted (Table III). Requisite
formulas and age specific incidence rates are provided as Annexure.
Table III
Incremental Cost-Effectiveness Estimates: Base- Case Analysis and Range
|
Activity |
Cost |
Measles |
Measles |
Discounted |
Costs per |
Costs per |
Costs per |
|
(INR) |
Cases |
Deaths |
DALYs |
case |
death |
discounted |
|
|
(upto 5 yr |
|
|
averted |
averted |
DALY averted |
|
|
of age) |
|
|
|
|
|
| Routine Immunization |
Unknown |
139982 |
3500 |
125349 |
|
|
|
| Routine plus SIA |
Unknown + |
74504 |
1863 |
66712 |
|
|
|
|
25177095 |
|
|
|
|
|
|
| Incremental Values |
|
|
|
|
|
|
|
| Base Case |
25177095 |
65479 |
1637 |
58638 |
INR 385 |
INR 15381 |
INR 430 |
| C.I. |
|
54986- |
1375- |
49464- |
INR 223- |
INR 8903- |
INR 249- |
|
|
113119 |
2828 |
101292 |
406 |
16212 |
453 |
Discussion
Measles vaccination in India when administered as a
supplementary immunization strategy, is a cost-effective intervention
compared to the status quo of measles vaccination by routine immunization
alone, assuming a willingness-to-pay of US $950 per DALY averted. At an
ICER of INR 430 per DALY averted, the result highly favors program
implementation considering that the World Bank has described any activity
which costs less than US$ 100 per DALY saved as highly cost effective for
developing nations. The results achieved in this study are comparable to
the lowest values in comparative cost effective analysis(17). There are
three reasons for the favorable cost effectiveness ratio. First, vaccine
coverage rates under routine immunization are low. Second, the incidence
of measles in the Indian population is high. Lastly, the vaccination cost
per child is quite low.
Estimates used in this study were from studies in
settings from all over India and also some studies from other developing
countries. To account for imprecision to minimize favoring SIA this paper
used conservative estimates, limited the rate of complications due to
measles to upto 5 years of age, and applied large ranges of sensitivity
around the base estimates for sensitivity analysis. Under conservative
assumptions of invasive measles infection, the introduction of
supplementary immunization activity appears to be a very good investment,
especially in states with lower than national average (Bihar, Jharkhand,
Uttar Pradesh, Rajasthan), which are also among the most populated states
in India with a combined population in the 0-6 year age group of 160
million (National Census, 2001).
If routine immunization coverage for measles can be
expanded to include a second dose of the vaccine as in other developed
countries, it will prove to be even more cost effective than SIA in
lowering the morbidity due to measles. It would prevent the extra costs of
manpower, material, IEC, and community mobilization required for SIA.
However, the effectiveness would depend upon the vaccination coverage
rate. The coverage rates could vary and a coverage rate for the second
dose is more likely to be lower than that for the primary dose, even if it
increases the coverage rate for a single dose of the vaccine (given the
fact that other vaccines with multiple doses (e.g. DPT, OPV) show a
similar pattern). In contrast, coverage levels are almost always higher in
SIA as compared to the routine immunization coverage, as witnessed in
earlier programs. Hence, strengthening of the routine immunization
coverage for the first dose should be the primary strategy in dealing with
measles morbidity, with the second dose of vaccine being included in the
routine immunization program only in districts which have shown
consistently high levels of coverage for the first dose and have the
resources to sustain the strategy. This is also consistent with the
Measles mortality reduction India strategic plan 2005-2010(1). Thus, while
second dose through routine immunization would be a good strategy for high
coverage districts, SIA can be a good strategy to supplement primary
coverage in low coverage districts.
Future initiatives should also be focused on
strengthening health systems to improve cold chain maintenance and
maintain vaccine efficacy, and increase vaccine coverage levels through
routine immunization activity.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Measles morbidity and mortality rates in India are high due to
poor measles vaccine coverage.
What This Study Adds?
• A supplementary immunization activity for
measles, although costlier than introducing a second dose through
routine immunization, is a cost effective option for lowering
morbidity and mortality due to measles in districts with coverage
lower than the National average.
|
Annexure
Population at risk at a certain age = Total
population – (children who have had developed measles + children who
have developed immunity following immunization.
Total Cases upto 5 years of age =
S (Population at risk at a
certain age)*(Age specific incidence rate upto 5 years of age).
Total Deaths = Total Cases upto 5 years of
age*Probability of death following measles.
Total DALY’s = S
{Probability of developing a complication following measles*
disability weight associated with a complication* age weighted
duration of a disability/ (1+r) ^n}
References
1. Measles mortality reduction. India Strategic Plan
2005-2010. Ministry of Health and Family Welfare, Government of India.
http://www.whoindia.org/LinkFiles/Measles_Measles pdf.pef. Accessed on
November 13, 2008.
2. Department of Family Welfare, Ministry of Health and
Family Welfare. Multi Year Strategic Plan 2005-2010. Universal
Immunization Programme. Government of India. January 2005. From: http://www.whoindia.org/LinkFiles/Routine_
Immunization_MYP_PDF_(o5_July_05_Final.pdf. Accessed on August 18, 2008.
3. de Quadros CA, Olive JM, Hersh BS, Strassburg MA,
Henderson DA, Brandling-Bennett D, et al. Measles elimination in the
Americas: evolving strategies. JAMA 1996: 275: 224-229.
4. TreeAge Software. Williamstown; MA: 2003.
5. Microsoft Corporation. Excel, Inc. Redmond; WA:
2003.
6. Zhou F, Reef S, Massoudi M, Papania MJ, Yusuf HR,
Bardenheier B, et al. An economic analysis of the current universal
2-dose measles-mumps-rubella vaccination program in the United States.
Infec Dis 2004; 189: S131-145.
7. Bareilly District Office, National Polio
Surveillance Program.
8. International Institute for Population Sciences (IIPS)
and Macro International. 2007. National Family Health Survey (NFHS-3)
India, Mumbai: IIPS; 2005-2006.
9. Singh J, Sharma RS, Verghese T. Measles mortality in
India: a review of community based studies. J Commun Dis 1994: 26:
203-214.
10. Sood DK, Kumar S, Singh S, Sokhey J. Adverse
reactions after measles vaccination in India. Natl Med J India 1995; 8:
208-210.
11. Sanford R, Kimmel MD. Vaccine adverse events:
separating myth from reality. Am Fam Phys 2002; 66: 2113-2120.
12. Dubey AP, Banerjee S. Measles, mumps, rubella
vaccines. Indian Pediatr 2003; 7: 579-584.
13. Puri A, Gupta VK, Chakravarti A, Mehra M. Measles
vaccine efficacy evaluated by case reference technique. Indian Pediatr
2002; 39: 556-560.
14. Yadav S, Thukral R. Chakarvarti A. Comparative
evaluation of measles, mumps and rubella vaccine at 9 and 15 months of
age. Indian J Med Res 2003; 118: 183-186.
15. Lopez AD. Mathers CD, Ezzatii M, Jamison DT, Murray
CJL. Global burden of disease and risk factors. 2006. The International
Bank for Reconstruction and Deveolopment. The World Bank. Available from:
http://files.dep2.org/pdf/GBD/GBD. Assessed on August 18, 2008.
16. Disease Control Priorities Project. Uncertainty and
Sensitivity Analysis for Burden of Disease and Risk Factors Estimates.
Available from: http://www.dep2.org/pubs/GBD/5. Accessed on August 18,
2008.
17. Griffiths UK, Wolfson LJ, Quaddus A, Younus M,
Hafiz RA. Incremental cost effectiveness of supplementary immunization
activities to prevent neonatal tetanus in Pakistan. Bull Wld Health
Organization 2004; 82: 643-651.
18. Pongrithsukda V, Phonboon K, Manunpichu K. Measles
associated diarrhea in North Eastern Thailand. South East Asian J Trop Med
Pub Health 1986; 97: 43-47.
19. Development N, Mala N, Ashamed SS. Shankar VJ.
Measles associated diarrhea and pneumonia in south India. Indian Pediatr
1994: 31; 35-42.
20. Bhaskaram P. Measles and malnutrition. Indian J Med
Res 1995; 102: 195-199.
21. Semba RD, Bloem MW. Measles blindness. Surv
Ophthalmol 2004; 19: 243-255.
22. Ray SK, Mallik S, Munsi AK, Mita SP, Baur B, Kumar
S. Epidemiological study of measles in slum areas of Kolkata. Indian
Pediatr 2004; 7: 583-586.
|
|
|
 |
|

