-thalassemia major
was made after a complete hemolytic work-up.
Fragmented Red Cell (schistocytes) Count (FRC)
Schistocytes are red blood cell fragments, which are
usually produced as a result of mechanical damage within the
vasculature. As per recommendations of ‘International Council for
Standardization in Haematology’ (ICSH) [6], schistocytes are defined as
cell fragments which are smaller than a normal RBC with sharp angles and
straight borders, or may be small crescent shaped. Various other
morphological forms, like helmet cells, keratocytes, or microspherocytes
are also included under this category. Recent hematology analyzers (Advia
2120i and Sysmex XN) have been equipped with various proprietary
algorithms to quantify as well as flag these cells by the term
‘fragmented red cells’ (FRC). Studies have shown a good morphological
correlation with these automated counts [7]. This parameter has been
shown to have good negative predictive value but a low specificity;
hence, all positive cases need to be reviewed on smears. The normal
upper limit for a healthy adult is generally set as <1%, though there is
no consensus [6]. In case of newborns, schistocytes are more frequently
seen with a range of 1.4-1.9% in term babies and 4.9-5.5% in preterm
babies [6]. The prime value of detecting significant numbers of
schistocytes in pediatric patients is to detect an underlying condition
resulting in thrombotic microangiopathy (TMA). In a neonate who presents
with jaundice and thrombocytopenia along with an FRC count of >1% (>5%
in preterm), an underlying disseminated intravascular coagulation (DIC)
secondary to perinatal asphyxia, infection or sepsis should be
considered. Besides, other conditions causing such manifestations may be
neonatal hemolytic uremic syndrome, congenital form of thrombotic
thrombocytopenic purpura (ADAMTS13 deficiency), homozygous protein C
deficiency or a giant hemangioma/vascular tumor [8]. There are certain
other conditions such as burns, thalassemia syndromes, megaloblastic
anemia, congenital sideroblastic and dyserythropoietic anemias and
prolonged iron deficient states where schistocytes are found along with
other poikilocytes, but associated thrombocytopenia is usually not seen,
and clinical symptomatology is different from that seen in TMA or DIC
[6] (Fig. 1).
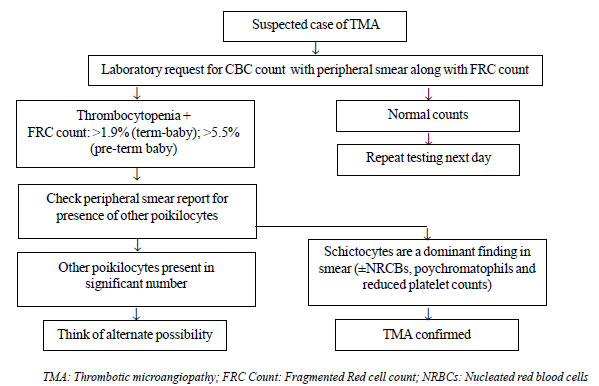 |
|
Fig. 1 Approach to a suspected case of
Thrombotic Microangiopathy (TMA) using the FRC count.
|
Immature Reticulocyte Fraction (IRF)
Immature reticulocyte fraction (IRF) is defined as
the fraction of most immature forms of reticulocyte to the total number
of reticulocytes. These reticulocytes have the maximum amount of RNA
which can be detected using various RNA binding fluorescent dyes by HAs.
HAs usually discriminate the reticulocytes into three population groups
based on the intensity of fluorescence; high, medium and low
fluorescence reticulocytes (HFR, MFR and LFR). IRF is the sum total of
HFR and MFR. Different instruments uses different dyes for their
identification; hence, there are different reference ranges for it (Table
I). IRF value provides an assessment of the reticulocyte maturation
and hence, the degree of effective erythropoiesis. For the diagnosis of
various types of anemias, IRF serves as an adjunct to total reticulocyte
count but is usually not of much help alone in differentiating them all
(Fig. 2). The scenarios in which IRF can be applied
clinically are summed up in Box 2.
|
Box 2 Immature Reticulocyte Fraction:
When to Choose and Where to Use?
• Evaluation of cases of anemia.
• Marker to assess response to iron or
vitamin-B12/folate supplementation in nutritional anemias and to
monitor erythropoietin (EPO) therapy response as it rises much
earlier before the total reticulocyte count [2].
• Alternative for absolute neutrophil count
(ANC) for monitoring recovery following bone marrow transplant,
as it starts to rise 5-7 days post bone marrow transplant and
reaches >10% at 10-14 days and is not affected by infections
which are common in such settings [9-10].
• Useful indicator of impaired bone marrow
function following chemotherapy-induced bone marrow aplasia in
cancer patients [11].
|
| |
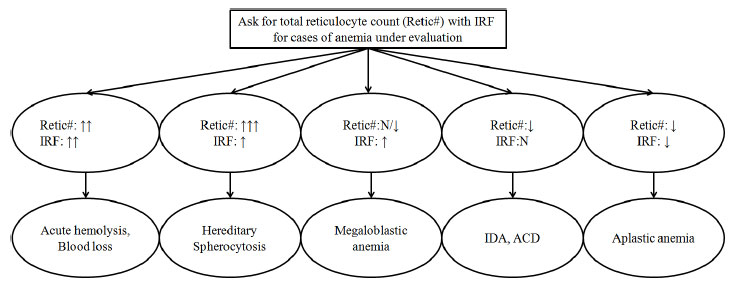 |
|
Fig. 2 Utility of total reticulocyte
count and Immature Reticulocyte fraction (IRF) for cases of
anemia under evaluation.
|
New Parameters for Assessing Functional Iron
Deficiency (FID)
Functional iron deficiency (FID) is defined as a
state arising due to non-availability of iron for the developing
erythroid precursors in the bone marrow in the presence of adequate body
iron stores, ultimately resulting in anemia. This is attributed to
trapping of iron within the reticuloendothelial system in
inflammatory/infectious conditions or in chronic kidney disease patients
undergoing regular dialysis sessions. Traditionally, this state was used
to be detected with the use of iron profile (serum ferritin, percentage
saturation of transferrin). However, their values are often deranged if
confounded by inflammation, cancer or infections. Hence, there was a
need to identify reliable parameters which could detect the iron
deficiency at the very stage of its incorporation into the erythroid
precursors (reticulocytes) and at the same time not being affected by
the confounders.
Modern-day hematology anlyzers have come up with many
new parameters to better characterize the reticulocytes which remain in
circulation for two to three days and hence are a better indicator of
early changes for the iron-restricted erythropoiesis. The most widely
used and clinically important parameters amongst these include, the mean
content of hemoglobin within the reticulocytes [CHr-mean reticulocyte
hemoglobin content as given on Siemens Advia analyzer] or its
equivalent; [Ret-He-reticulocyte hemoglobin equivalent as given on
Sysmex analyzer] and percentage hypochromic cells (% HRC; Siemens Advia,
Sysmex XE series, CELL-DYN Sapphire) or its equivalent low hemoglobin
density (LHD%; Beckman coulter).
Studies have found a good correlation among CHr and
Ret-He as they can provide the status of functional iron available for
the erythropoiesis during the last 3-4 days [12-13]. Together with % HRC
or % LHD parameter (defined as RBC’s with cellular Hb <28g/dL and volume
< 60 fL), they are termed as iron restricted erythropoiesis (IRE)
markers, and are helpful in various clinical settings. The reference
range for adults for Ret-He is approximately 28-35 pg (below 28 pg is
considered iron deficient) and % HRC is <2.5% [13]. The main clinical
utility of these IRE markers is in differentiating actual iron
deficiency anemia (IDA) versus FID state (IDA- CHr or Ret-He <25
pg and % LHD >6% versus FID- CHr or Ret-He <28 pg and % LHD
>2.5%). It is also useful in planning need for iron supplementation in
cases of anemia of chronic disease (ACD) or for parenteral iron in
chronic kidney disease (CKD) cases on erythropoietin stimulating agents
(ESA) (when CHr or Ret-He <28 pg and % LHD >6% with a reduced to normal
ferritin level) [15] (Fig. 3).
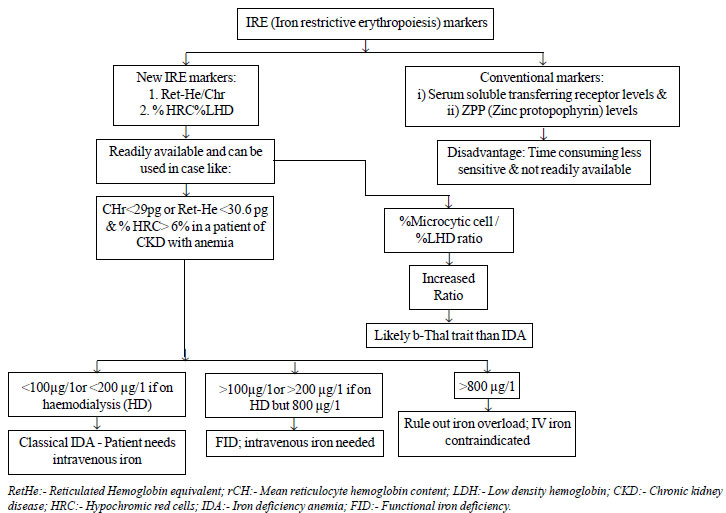 |
|
Fig. 3 Utility of Iron Restricted
erythropoiesis (IRE) markers in clinical case scenarios.
|
% LHD along with % microcytic cells (given by Sysmex
XN-1000 series), is also used to distinguish between IDA and
-thalassemia trait
or other hemoglobinopathies [17].
WBC Parameters
White Blood Cell Volume, Conductivity and Scatter
(VCS)
Volume, Conductivity and Scatter
(VCS) technology of the Beckman Coulter series is an approach to WBC
analysis where in addition to numerically quantifying and
sub-classifying these cells, it also yields a large amount of
information on their physical, electrical, optical and hence, structural
properties. The numerical VCS data or coordinates are visualized
graphically in the form of a 3-D cube. A total of 24 parameters (mean
positions on the 3-axes of Figure, and standard deviation for each cell
type) are thus available with every routine differential leukocyte count
without any further analyses or increased cost, and regardless of
clinical suspicion. Various authors have utilized VCS parameters (mean
neutrophil volume, MNV) for early identification of neonatal sepsis by
formulating the regression equation which combines other laboratory
parameter [18-19]. Other uses of VCS technology are detection of
chorio-amnionitis, malaria and dengue as well as malignancies like
myelodysplastic syndromes, myeloproliferative neoplasms, acute leukemias
and lymphoproliferative disorders [20]. Various authors have devised
their own algorithmic approaches so as to characterize a particular
disease state.
Malaria factor has been derived using VCS parameters
like SD of the mean volume for lymphocytes (MLV-SD) and Monocytes
(MMV-SD) for the possible presence of malarial parasites. A cut-off
value for the Malaria Factor greater than 3.7 is an indicator of malaria
infection with the specificity of 94% and sensitivity 98% [21].
Immature Granulocytes (IGs)
This parameter identifies and quantifies immature
myeloid cells which combine promyelocytes, myelocytes and metamyelocytes
and helps overcome the possibility of missing these cells in a manual
100-cell differential leukocyte count, especially in leukopenic
patients. Their presence in the peripheral blood is indicative of a
systemic inflammation and sepsis, a hematological disorder like
myeloproliferative neoplasm or acute myeloid leukemia, or a bone marrow
infiltrative disorder (where peripheral blood smear may show
leuco-erythroblastic picture). In fact, an immature to total (I: T)
granulocytic cell ratio of >0.2 or an immature to mature (I:M)
granulocytic cell ratio of >0.3 is a 100% sensitive marker for diagnosis
of sepsis in an appropriate clinical setting [22].
Atypical/Immature Lymphocytes
The detection and quantification of atypical
lymphocytes has been provided with the Sysmex XE series (High
fluorescent lymphocytes, HFL), Horiba Pentra (Atypical lymphocytes,
ALY%) and Siemens Advia 2120 (Large unstained cells, %LUC). This
parameter can quantify various morphologies of atypical lymphocytes like
activated lymphocytes in viral infections (e.g., in infectious
mononucleosis), lymphoma cells as well as small blasts. Hence, it can
also be used in the monitoring of sepsis because of viral infections
[20].
Neutrophil Granulation (NEUT-X/NEUT-Y)
NEUT-X is a measure of granularity of neutrophils
based on their side-scatter property whereas NEUT-Y indicates cellular
content of nucleic acid and protein. These parameters are provided by
Sysmex XN/XE series. Both these parameters have been shown to have an
increased value in sepsis and a low value in cases of myelodysplastic
syndromes or myelodysplastic/myeloproliferative neoplasms (chronic
myelomonocytic leukemia) [23,24].
Hematopoietic Progenitor Cell Count
The quantitative hematopoietic progenitor cell (HPC)
count is offered by Sysmex XE-2100 and XN-2000. This can be used for
determining the optimal time for cell harvest in cases of hematopoietic
stem cell transplant. HPC count provided by the instrument is
substantially equivalent to CD34+ count by flow cytometry [25].
Platelet Parameters
Mean Platelet Volume (MPV)
Mean platelet volume is derived from the impedance
platelet size distribution curve. MPV is calculated by dividing the
plateletcrit with platelet count. The normal reference range is 7-12 fL.
It is provided by almost all latest analyzers. A high MPV is seen in
inherited macrothrombocytopenia (like Bernard-Soulier Syndrome) and
myeloproliferative neoplasms. MPV is also high in immune mediated
pathologies of thrombocytopenia like immune thrombocytopenic purpura
(ITP) as compared to primary bone marrow pathologies resulting in
thrombocytopenia [26]. As large platelets are functionally more active
than smaller ones, high MPV has been observed to predict higher risk of
and following myocardial infarction and/or stroke in combination with
other risk factors in various studies, especially in pediatric cases
with type I diabetes mellitus [27].
Reticulated Platelets and Immature Platelet Fraction
(IPF)
Reticulated platelets are the young platelets with a
higher RNA content. HAs like Sysmex XE/XN series, Abbott CELL-DYN
Sapphire and BC-6800 Mindray quantify the reticulated platelets and IPF.
The reference range is 1.1-6.1% of the platelet count [28]. IPF is
raised in patients with peripheral destruction of platelets (ITP and
thrombotic thrombocytopenic purpura, TTP) and is normal or low in
patients with bone marrow failure [28]. IPF is also shown to be useful
following a peripheral blood stem cell transplant where it has been
shown to increase 1-2 days prior to the increase of platelet count [29].
They are increased in the circulation following recovery of
thrombopoiesis in dengue fever and it has been shown in studies that a
single value of >10% is indicative of platelet recovery within 24-48
hours, thereby reducing load of unnecessary platelet transfusions in
clinical setting [30].
Case scenario 2
A 10-year-old girl presented with history of
high-grade fever, arthralgia along with petechial rash and nose bleed
for 2 days. There was an ongoing outbreak of dengue fever in the area
and the resident doctor ordered complete blood counts (CBC) along with
NS1 antigen test for dengue virus. Investigations revealed
thrombocytopenia of 15x10
1. Teixeira C, Barbot J, Freitas MI. Reference values
for reticulocyte parameters and hypochromic RBC in healthy children. Int
J Lab Hematol. 2015;37:626-30.
2. Piva E, Brugnara C, Spolaore F, Plebani M.
Clinical utility of reticulocyte parameters. Clin Lab Med.
2015;35:133-63.
3. Hwang DH, Dorfman DM, Hwang DG, Senna P,
Pozdnyakova O. Automated nucleated RBC measurement using the sysmex
XE-5000 hematology analyzer: Frequency and clinical significance of the
nucleated RBCs. Am J Clin Pathol. 2016;145:379-84.
4. Stachon A, Segbers E, Holland-Letz T, Kempf R,
Hering S, Krieg M. Nucleated red blood cells in the blood of medical
intensive care patients indicate increased mortality risk: a prospective
cohort study. Crit Care. 2007;11:R62.
5. Otsubo H, Kaito K, Asai O, Usui N, Kobayashi M,
Hoshi Y. Persistent nucleated red blood cells in peripheral blood is a
poor prognostic factor in patients undergoing stem cell transplantation.
Clin Lab Haematol. 2005;27:242-6.
6. Zini G, d’Onofrio G, Briggs C, Erber W, Jou JM,
Lee SH, et al. ICSH recommendations for identification,
diagnostic value, and quantitation of schistocytes. Int J Lab Hematol.
2012;34:107-16.
7. Saigo K, Jiang M, Tanaka C, Fujimoto K, Kobayashi
A, Nozu K, et al. Usefulness of automatic detection of fragmented
red cells using a hematology analyzer for diagnosis of thrombotic
microangiopathy. Clin Lab Haematol. 2002;24:347-51.
8. Christensen RD, Yaish HM, Lemons RS. Neonatal
hemolytic jaundice: morphologic features of erythrocytes that will help
you diagnose the underlying condition. Neonatology. 2014;105:243-9.
9. Noronha JF, De Souza CA, Vigorito AC, Aranha FJ,
Zulli R, Miranda EC, et al. Immature reticulocytes as an early
predictor of engraftment in autologous and allogeneic bone marrow
transplantation. Clin Lab Haematol. 2003;25: 47-54.
10. d’Onofrio G, Tichelli A, Foures C, Theodorsen L.
Indicators of haematopoietic recovery after bone marrow transplantation:
the role of reticulocyte measurements. Clin Lab Haematol. 1996;18:45-53.
11. Luczynski W, Ratomski K, Wysocka J,
Krawczuk-Rybak M, Jankiewicz P. Immature reticulocyte fraction (IRF)–an
universal marker of hemopoiesis in children with cancer? Adv Med Sci.
2006;51:188-90.
12. Brugnara C, Schiller B, Moran J. Reticulocyte
hemoglobin equivalent (Ret He) and assessment of iron-deficient states.
Clin Lab Haematol. 2006;28:303-8.
13. Garzia M, Di Mario A, Ferraro E, Tazza L, Rossi
E, Luciani G, et al. Reticulocyte hemoglobin equivalent: An
indicator of reduced iron availability in chronic kidney diseases during
erythropoietin therapy. Lab Hematol. 2007;13:6-11.
14. Schoorl M, Linssen J, Villanueva MM, NoGuera JA,
Martinez PH, Bartels PC, et al. Efficacy of advanced
discriminating algorithms for screening on iron-deficiency anemia and â-thalassemia
trait: a multicenter evaluation. Am J Clin Pathol. 2012;138:300-4.
15. Buttarello M. Laboratory diagnosis of anemia: are
the old and new red cell parameters useful in classification and
treatment, how? Int J Lab Hematol. 2016;38:123-32.
16. Nair SC, Arora N, Jain S, Inbakumar D, Mammen J,
Sitaram U. Mean reticulocyte volume enhances the utility of red cell
mean sphered cell volume in differentiating peripheral blood spherocytes
of hereditary spherocytosis from other causes. Indian J Pathol Microbiol.
2015;58: 307-9.
17. Ng EH, Leung JH, Lau YS, Ma ES. Evaluation of the
new red cell parameters on Beckman Coulter DxH800 in distinguishing iron
deficiency anaemia from thalassaemia trait. Int J Lab Hematol.
2015;37:199-207.
18. Raimondi F, Ferrara T, Maffucci R, Milite P, Del
Buono D, Santoro P, et al. Neonatal sepsis: a difficult
diagnostic challenge. Clin Biochem. 2011;44:463-4.
19. Bhargava M, Saluja S, Sindhuri U, Saraf A, Sharma
P. Elevated mean neutrophil volume+CRP is a highly sensitive and
specific predictor of neonatal sepsis. Int J Lab Hematol.
2014;36:e11-14.
20. Briggs C. Quality counts: new parameters in blood
cell counting. Int J Lab Hematol. 2009;31:277-97.
21. Briggs C, Da Costa A, Freeman L, Aucamp I,
Ngubeni B, Machin SJ. Development of an automated malaria discriminant
factor using VCS technology. Am J Clin Pathol. 2006;126:691-8.
22. Khair KB, Rahman MA, Sultana T, Roy CK, Rahman
MQ, Ahmed AN. Early diagnosis of neonatal septicemia by hematologic
scoring system, C-reactive protein and serum haptoglobin. Mymensingh Med
J. 2012;21:85-92.
23. Furundarena JR, Araiz M, Uranga M, Sainz MR,
Agirre A, Trassorras M, et al. The utility of the Sysmex XE-2100
analyzer’s NEUT-X and NEUT-Y parameters for detecting neutrophil
dysplasia in myelodysplastic syndromes. Int J Lab Hematol.
2010;32:360-6.
24. Luo Y, Lin J, Chen H, Zhang J, Peng S, Kuang M.
Utility of neut-X, neut-Y and neut-Z parameters for rapidly assessing
sepsis in tumor patients. Clin Chim Acta. 2013;422:5-9.
25. Peerschke EI, Moung C, Pessin MS, Maslak P.
Evaluation of new automated hematopoietic progenitor cell analysis in
the clinical management of peripheral blood stem cell collections.
Transfusion. 2015;55:2001-9.
26. Ntaios G, Papadopoulos A, Chatzinikolaou A,
Saouli Z, Karalazou P, Kaiafa G, et al. Increased values of mean
platelet volume and platelet size deviation width may provide a safe
positive diagnosis of idiopathic thrombo-cytopenic purpura. Acta
Haematol. 2008;119:173-7.
27. Turfan M, Erdogan E, Ertas G, Duran M, Murat SN,
Celik E, et al. Usefulness of mean platelet volume for predicting
stroke risk in atrial fibrillation patients. Blood Coagul Fibrinolysis.
2013;24:55-8.
28. Briggs C, Kunka S, Hart D, Oguni S, Machin SJ.
Assessment of an immature platelet fraction (IPF) in peripheral
thrombocytopenia. Br J Haematol. 2004;126:93-9.
29. Zucker ML, Murphy CA, Rachel JM, Marlinez GA,
Abhyanker S, Mc Guirk JP, et al. Immature platelet fraction as a
predictor of platelet recovery following hematopoietic progenitor cell
transplantation. Lab Hematol. 2006;12:125-30.
30. Dadu T, Sehgal K, Joshi M, Khodaiji S. Evaluation
of the immature platelet fraction as an indicator of platelet recovery
in dengue patients. Int J Lab Hematol. 2014;36:499-504.
31. Zhang KJ, Lu QY, Li P, Zhang P, Niu XQ.
[Significance of platelet parameters and lactate dehydrogenase level in
differential diagnosis for thrombocytosis]. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 2010;18:972-5.
32. Tang J, Gao X, Zhi M, Zhou HM, Zhang M, Chen HW,
et al. Plateletcrit: a sensitive biomarker for evaluating disease
activity in Crohn’s disease with low hs-CRP. J Dig Dis 2015;16:118-24.

