|
|
|
Indian Pediatr 2016;53: 388-390 |
 |
Serum Phenobarbitone Levels in Term and
Near-Term Neonates with Seizures
|
|
Sanober Wasim, Amit Upadhyay, Monica Roy, Pranjali
Saxena and #Neelam
Chillar
From Department of Pediatrics, Lala Lajpat Rai
Memorial Medical College, Meerut; and #Institute of Human Behavior and
Allied Sciences, New Delhi; India.
Correspondence to: Dr Amit Upadhyay, Head, Department
of Pediatrics, LLRM Medical College, Meerut, India.
Email: [email protected]
Received: June 10, 2013;
Initial review: October 21, 2014;
Accepted: March 16, 2016.
|
Objective: To evaluate serum phenobarbitone levels in neonates with
seizures and to evaluate the effect of repeated loading dose on serum
phenobarbitone levels.
Methods: In this prospective observational study
conducted in a tertiary care centre of Northern India during 2011- 2012,
99 neonates admitted with seizureswere included.Serum phenobarbitone
levels in neonates with seizures at 20 minutes and 12 hours after the
first loading dose of phenobarbitone were measured.
Results: Serum phenobarbitone levels [mean (SD)]
at 20 min and 12 hours was 27.3 (28.4) µg/mL and 23 (19.1) µg/mL,
respectively (P=0.07). The mean serum phenobarbitone levels 12
hours after the loading dose, and proportion of neonates with toxic
levels increased with each loading dose of intravenous phenobarbitone.
Conclusion: Monitoring of serum level of
phonobarbitone may not be essential because seizure control in neonates
appears to be independent of whether serum level is subtherapeutic,
therapeutic or toxic range.
Keywords: Anticonvulsants, Drug Levels, Neonatal convulsions,
Toxicity.
|
|
S
eizure is the most common neurological emergency
in the neonatal period. Most clinical guidelines recommend empirical
treatment with phenobarbitone as the first line drug for neonatal
seizures [1-3]. While treating seizures, the value of periodic
monitoring of plasma levels of phenobarbitone has been recognized on the
basis of the known relationship between plasma levels and brain tissue
levels [4]. Since phenobarbitone has a narrow therapeutic index and wide
inter-patient variability in its utilization, there have been concerns
about the dosing schedule [5,6]. The distribution, metabolism and
excretion of phenobarbitone may show variability, depending upon the
ethnicity, seizure etiology and condition of various body organs
[5,7,8]. There is a paucity of studies reporting phenobarbitone levels
in neonatal seizures from Indian subcontinent. This study was planned to
evaluate the serum phenobarbitone levels in neonates with seizures, and
to evaluate the effect of repeated loading dose on serum phenobarbitone
levels.
Methods
This was a prospective observational study, conducted
in a level II neonatal intensive care unit of a teaching hospital in
Northern India between July 2011 to September 2012. This study was
initiated after getting approval from the institutional ethics
committee.
Study population included neonates with clinically
apparent seizures not responding to treatment of hypoglycemia or
hypocalcemia and (i) weight more than 2 kg; (ii) gestation
>34 weeks; and (iii) post-natal age less than 4 weeks. Neonates
with major congenital malformations and those requiring intubation and
ventilation at the time of first seizure were excluded from the study.
Asphyxia was defined as presence of any one of the
following: (i) Intramural babies: APGAR score less than 3 at 1
minute and arterial pH <7 at the time of admission, (ii)
Extramural: history of delayed cry >3 mins after birth. Each neonate was
enrolled after obtaining written informed consent of the parent.
Neonatal seizures were treated with a loading dose of intravenous
phenobarbitone in dose of 20 mg/kg administered over a 20 min period at
a rate of 1 mg/kg/min. A responder was defined as an infant who remained
seizure-free for a period of 24 hours. For persistent seizures, infants
received second dose of phenobarbitone, levetiracetam, phenytoin and/or
midazolam as required, according to unit policy. Blood samples for
analysis of serum phenobarbitone levels were obtained 20 min (i.e.
soon after the first loading dose and before second dose of
anticonvulsant in case of non-responders) and 12 hours (before giving
first maintenance dose) after the completion of loading dose. Serum was
separated and stored at –20°C. Samples were analyzed by the CEDIA
Phenobarbital II assay (Microgenics, USA), an in vitro method
intended for the quantification of phenobarbitone in human serum by
spectrophotometry. The serum levels were classified as sub-therapeutic
(<10 µg/mL), therapeutic (10-40 µg/ mL) and toxic (>40 µg/ mL).
Analysis of continuous data with normal distribution
was done by unpaired t test and non-normally distributed data was done
by Mann-Whitney test. Mean of various outcomes were compared using
unpaired t-test. Repeated measure analysis was done for serum
phenobarbitone levels at two time points. Categorical data was compared
using chi square or Fischer exact test, as applicable. P value
less than 0.05 was considered significant.
Results
A total of 119 neonates were admitted with seizures,
of which 99 were included in the study. Three had hypoglycemic and 5 had
hypocalcemic seizures, two refused consent, 9 required ventilatory
support at the time of seizure and one had multiple congenital
malformations. The mean (SD) serum phenobarbitone level at 20 min and 12
hours was 27.3 (28.4) and 23.0 (19.1) mcg/mL, respectively (P=0.07)
(Fig. 1). Therapeutic serum levels were attained in only
51 (58%) neonates, while 23 (26%) had sub-therapeutic levels and 14
(16%) had toxic levels of serum phenobarbitone at 12 hours. Clinical
control of seizure after single loading dose of 20 mg/kg was achieved in
44 (44.4%) neonates. Proportion of seizure control in neonates with
serum phenobarbitone level in therapeutic, sub-therapeutic and toxic
range was 22 (46%), 8 (40%) and 8 (47%), respectively (P=0.8).
Time to reach full enteral feed and oral feed was comparable in neonates
achieving therapeutic, sub-therapeutic and toxic levels of serum
phenobarbitone. In neonates with hypoxic ischemic encephalopathy (HIE),
though the mean serum level at 20 min was higher in HIE stage 3 than
stage 1 or stage 2, the difference in serum levels in the three stages
was not significant (P=0.42). The mean serum phenobarbitone
levels 12 hours after the loading dose, and proportion of neonates with
toxic levels increased with each loading dose of intravenous
phenobarbitone (Table I).
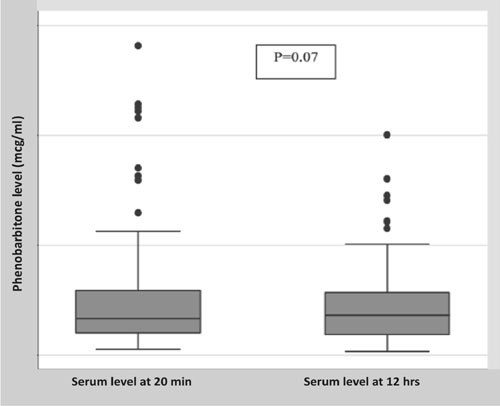 |
|
Fig. 1 Box and Whiskers plot
for serum phenobarbitone levels.
|
TABLE I Serum Phenobarbitone Levels According to Loading Dose Given
|
Loading dose
|
|
20 mg/kg |
30 mg/kg |
40 mg/kg |
| |
(n = 44) |
(n = 29) |
(n=6) |
|
Level at 12 hours (mg/mL); Mean (SD) |
19.3 (17) |
25.3 (16.3) |
48.9 (31.7) |
|
*Neonates with toxic levels at 12 h, n (%) |
4 (9) |
5 (17) |
4 (66) |
|
*P=0.003 for number with toxic levels in 40 mg/kg vs
20 mg/kg group. |
Discussion
Our study demonstrated that seizure control in
neonates was not different in those with subtherapeutic, therapeutic or
toxic levels of phenobarbitone. We also found that serum phenobarbitone
level after first loading dose of intravenous phenobarbitone was within
therapeutic range in only about 60% of babies.
In our study, clinical control of seizures occurred
in only 44.4% of neonates which was similar to some earlier studies
[9,10]. In a previous study at our center, we found a high response rate
of 72% in the phenobarbitone group; however, serum levels were not
analyzed [11]. Few studies have attempted to correlate serum
phenobarbitone levels with control of neonatal seizures. Jamie, et al.
[10] reported a direct relationship between serum concentration and
response to phenobarbitone. In our study, though the mean serum level
was slightly higher in the seizure control group as compared to group in
which seizures were not controlled, the difference was not statistically
significant. Jalling, et al. [12] reported that convulsion ceased
at serum levels between 12 and 30 mcg/mL, although there was a subgroup
of convulsing patients whose seizures could not be controlled despite
achievement of therapeutic levels. In our study, the minimum serum
phenobarbitone level at which seizure was controlled was 6 mcg/mL.
Ouvrier and Goldsmith [13] have also documented seizure control with
phenobarbitone levels in range of 7 to 15 mcg/mL.
There were some neonates with unexpectedly high or
low serum levels documented in our study. This variance in serum level
could also be due to the heterogeneity of post-natal age and etiology of
seizures, both of which have been reported to unpredictably affect serum
levels in earlier studies [5,7]. Researchers have documented more than
50% reduction in clearance of phenobarbitone in asphyxiated neonates,
compared to non-asphyxiated neonates, leading to higher serum levels in
the former [10,14]. This was in contrast to a study by Fischer, et al.
[15], who demonstrated lower levels in neonates with asphyxia, probably
due to the effect of chronic hypoxia, which may promote the metabolism
of phenobarbitone. In our study, we did not find any association of
asphyxia and serum levels.
Our study demonstrated that with each subsequent
loading dose of phenobarbitone after 20 mg/kg results in significant
increase in the number of neonates attaining toxic levels. Therefore,
alternative or second line drugs should probably be used, rather than
the repeated doses of phenobarbitone in neonates not responding to first
loading dose of phenobarbitone, especially if facility to monitor serum
levels are not there at the center of treatment. However, further
studies with more patients receiving multiple loading doses of
phenobarbitone are required.
A limitation of our study was that continuous EEG
monitoring was not done and abolition of electrical seizures could not
be documented.
We conclude that intravenous loading dose of
phenobarbitone at 20 mg/kg results in therapeutic levels in most
neonates, but both sub-therapeutic and toxic levels of phenobarbitone
are possible. Seizure control appears to be independent of whether serum
level is subtherapeutic, therapeutic or toxic range.
Contributors: SW: collected and compiled
the data for the study, and drafted the initial manuscript; AU:
conceptualized and designed the study, analyzed the data and finalized
the manuscript. He shall act as guarantor of paper. NC: performed the
serum phenobarbitone levels ; MR, PS: helped in collecting blood
samples, data and preparation of manuscript.
Funding: Partially funded by ‘Thesis/
Research grant’ of Indian Council for Medical Research (ICMR), New
Delhi, India.
Competing interest: None stated.
|
What This Study Adds?
• Repeated doses of phenobarbitone can lead
to toxic levels in neonates when given in widely recommended
doses of up to 40 mg/kg.
|
References
1. Neonatal seizures: After all these years we still
love what doesn´t work. Neurology. 2005; 64:776-7.
2. Kanhere S. Recent advances in neonatal seizures.
Indian J Pediatr. 2014;81:917-25.
3. Sankar JM, Agarwal R, Deorari A, Paul VK.
Management of neonatal seizures. Indian J Pediatr. 2010;77:1129-35.
4. Onishi S, Ohki Y, Nishimura Y, Itoh S, Isobe K, Hosoe
A, et al. Distribution of phenobarbital in serum, brain and other
organs from pediatric patients. Dev Pharmacol Ther. 1984;7:153-9.
5. Gal P, Toback J. The influence of asphyxia on
Phenobarbital dosing requirements in neonates. Dev Pharmacol Ther.
1984;7:145-52.
6. Ouvrier RA, Goldsmith R. Phenobarbitone dosage in
neonatal convulsions. Arch Dis Child. 1982;57:653-7.
7. Jones DP. Hypoxia and drug metabolism. Biochemical
Pharmacol. 1981;30:1019-23.
8. Klotz U. The role of pharmacogenetics in the
metabolism of antiepileptic drugs: Pharmacokinetic and therapeutic
implications. Clin Pharmacokinet. 2007;46:271-9.
9. Painter MJ, Pippenger C, Mac Donald H, Pitlick W.
Phenobarbitone and diphenylhydantoin levels in neonates with seizures. J
Pediatr. 1978;92:315-9.
10. Jamie T, Gal P. Rapid sequential phenobarbital
treatment of neonatal seizures. Pediatrics. 1989; 83:674-8.
11. Pathak G, Upadhyay A, Pathak U, Chawla D, Goel
SP. Phenobarbitone and phenytoin for treatment of neonatal seizures: An
open-label randomized controlled trial. Indian Pediatr. 2013;50:753-7.
12. Jalling B. Plasma concentration of phenobarbitone
in the treatment of seizures in newborns. Acta Paediat Scand.
1975;64:514-24.
13. Ouvrier RA, Goldsmith R. Phenobarbitone dosage in
neonatal convulsions. Arch Dis Child. 1982;57:653-7.
14. Gal P, Boer HR. Phenobarbital dosing in neonates
and asphyxia. Neurology. 1982; 32:788-9
15. Fischer JH, Lockman LA, Zaske D, Kriel R.
Phenobarbitone maintenance dose requirements in treating neonatal
seizures. Neurology. 1981;31:1042-44.
|
|
|
 |
|

