|
|
|
Indian Pediatr 2015;52:
403-408 |
 |
Health Related Quality of Life in Indian
Children with Cystic Fibrosis
|
|
Devika Kir, Sumita Gupta, George Jolly, *M Kalaivani,
Rakesh Lodha and SK Kabra
From Departments of Pediatrics and *Biostatistics,
All India Institute of Medical Sciences, New Delhi, India.
Correspondence to: Dr SK Kabra, Professor, Department
of Pediatrics, All India Institute of Medical Sciences,
New Delhi 110 029, India.
Email: [email protected]
Received: August 27, 2014;
Initial review: October 21, 2014;
Accepted: February 20, 2015.
|
Objective: This study was devised to translate Cystic Fibrosis
Questionnaire-Revised to Hindi and administer it to Indian children and
adolescents diagnosed with cystic fibrosis.
Design: Cross-sectional study.
Setting: This study was carried
out in cystic fibrosis patients attending Pediatric Chest Clinic of a
tertiary-care hospital in Northern India from July 2012 to December
2012.
Participants: 45 children (6-13
years) and their parents, and 14 adolescents. Patients with unstable
health in the past two weeks were excluded.
Intervention: Cystic Fibrosis
Questionnaire- Revised translated in Hindi was administered. Clinical
evaluation and scoring, throat swab cultures and spirometry were also
done during the same visit.
Main Outcome Measures: Health
Related Quality of Life scores were the primary measures, and clinical
scores, swab cultures and spirometry were secondary measures.
Results: Cronbach’s alpha ranged
from 0.020-0.863.The Factor analysis indicated that most
test-items correlated more with competing scales than the intended
scales. Convergence between self and proxy-rating was found to be
dependent on the domain. The Cystic Fibrosis Questionnaire- Revised
scores correlated well with clinical scores (r=0.65,P=0.011),
Pseudomonas spp culture data and pulmonary function tests. There was
an inverse relation between Health Related Quality of Life scores and
age at diagnosis (r=-0.339, P=0.02).
Conclusions: Hindi versions of
Cystic Fibrosis Questionnaire- Revised: Child, Adolescent and Parents’
instruments will act as an important step towards data on Health Related
Quality of Life of Indian patients with cystic fibrosis.
Keywords: HRQoL, Outcome, Severity, Validation.
|
|
C
ystic fibrosis (CF), a chronic pulmonary disorder
is increasingly being recognized in India [1-3]. To measure the quality
of life of children with CF various methods have been used, common
being: pulmonary function testing, growth parameters, clinical scoring,
radiological scoring etc. However, these may not correlate directly with
quality of life [4,5]. More recently Patient-reported outcomes such as
the generic ones like 36-Item Short Form Health Survey (SF-36), and the
Quality of Well-Being scale, and more specific ones like CFQ, CFQoL,
Cystic Fibrosis Questionnaire Revised (CFQ-R) have been developed and
have been validated to assess psychological wellbeing of the children
with CF through various life stages [6-8]. A Patient-reported outcome
instrument is defined as any measure of a patient’s health status that
is directly elicited from the patient and assesses how the patient
‘‘survives, functions or feels’’ in relation to his or her health
condition [9,10]. CFQ was the first CF-specific patient-reported outcome
that gained the form of CFQ-R after few revisions, following
psychometric testing [10-16]. The CFQ-R is the only patient-reported
outcome instrument with versions for both children and the caregivers.
It has been translated into 34 languages all over the world [17-21]. Our
objective was to use CFQ-R to assess Quality of Life (QoL) in Indian
children with CF.
Methods
This cross-sectional study was carried out in
children with CF attending Pediatric Chest Clinic of a tertiary care
hospital in Northern India from July 2012 to December 2012. Subjects
included children 6 years and above diagnosed as CF based on compatible
clinical features with sweat chloride values of >60 mEq/L on two
occasions or with two known identified mutations. Consenting families
with children with their parents, and adolescents with ability to read
and write in Hindi, were included in the study. Subjects were excluded
from the study if they had an unstable health or missed school due to an
exacerbation in the past two weeks.
Disease severity was quantitated in three domains
viz., general activity of the child, findings on physical
examination, and nutrition of the child. Clinical scores were generated
out of 25 for each domain where maximum score was 100 and minimum score
was 0. A scaled clinical CF score [2,22] was calculated as previously
described. Other assessments done included throat swab cultures and
spirometry evaluation.
Health Related Quality of Life (HRQoL) was assessed
using the English version of CFQ-R. The English CFQ-R has three
formats; Child version (6-13) is a self-rating questionnaire comprising
of 35 test items and 8 domains, Adolescents version (14 and above)
containing 50 test items and 12 domains, while the Parents’ version is a
proxy rating consisting of 44 test items and 11 domains applicable only
for children 6-13 years of age. Adolescent’s version does not require
any proxy rating. The questions had to be answered keeping in mind the
physical and psychological health status over the two weeks preceding
the day of administration of the questionnaire. The questionnaire was
culturally adapted and translated in Hindi for use in Indian population.
It was translated from English to Hindi and back-translated by the two
translators independently. The words found misfit after back-
translation were substituted and agreed upon by both the translators.
Children aged 6-11 years were questioned by the interviewer and were
presented with options on flash cards. The chosen option was pointed out
by the child to the interviewer. The questionnaires were administered to
the patients and the parents in separate rooms, to avoid parents
influencing patients’ answers or vice-versa. All the other formats were
self-administered with an initial introductory brief by the
investigator. After completion by the patient/parent, any missing
answers were searched for by the investigator and the participant was
informed, eliminating most missing answers. It took about 10-15 min to
complete the questionnaire. Scaled scores for each domain were
calculated as described in the English CFQ-R.
The present study was approved by the Ethics
Committee of All India Institute of Medical Sciences. In all cases,
informed consent was obtained from at least one parent, and assent from
the child. Permission to use and translate English CFQ-R was obtained
from the authors.
Statistical analysis was conducted using the SPPS
20.0. Characteristics of study subjects including demographic profile,
clinical characteristics, PFT results, and Pseudomonas spp. on
culture results were analyzed. For reliability, internal consistency
was calculated using Cronbach’s alpha [23] for each domain in all
three questionnaire categories, viz., 6-13 years,14 and above and
parents’ version with a cutoff of 0.6 for reliability, before and after
deletion of the unreliable items.
Construct validity was evaluated by Factor analysis
(Principal Component Analysis, PCA) [24,25] and the Scree plot is
presented to test the given structure of the English CFQ-R. The Rotated
Component Matrix shows the domains in which the each individual test
items should be placed according to Factor analysis. The point at which
the plot dips down in the Scree plot marks the maximum number of
factors (domains) suggested by the analysis.
The difference in the mean HRQoL score was tested
between sex, spirometry findings and Pseudomonas spp culture
results using student’s t-test for independent samples. The subjects
were divided into four groups based on FEV1 percent predicted, FVC
percent predicted, PEF percent predicted: less than 40, 40-59, 60-79 and
³80. In case
of more than two groups, one way ANOVA was used for comparison
for parametric data and Kruskal-Wallis H for non-parametric data. We
used Bonferroni post-hoc test with alpha set at 0.05.Correlation between
clinical scores, age, and age at diagnosis, with HRQoL scores were
calculated by Pearson’s/Spearman’s correlation coefficient. The
convergent validity of the Child and Parent versions were calculated by
Intra Class Correlation. P value less than 0.05 was considered
statistically significant.
Results
A total of 59 (planned sample size was 60 but 1
patient was included twice) patients with CF were studied with 76% (45)
belonging to age group 6-13. The mean age (SD) of our study population
was 11.5 years (4.5), and 61% of the participants were boys. The mean
age (SD) of diagnosis was 5.7 years (4.5) (range 6 mo- 19 y). 28.8 %
(17) of all subjects were positive for Pseudomonas spp. on
culturing throat swab samples, out of which 16 belonged to the age group
6-13 and only 1 to the adolescent group. The average scaled clinical
scores were 78.5 (out of 100), children aged 6-13 scored an average of
80 percent (13.8 and adolescents aged 14 and above scored a mean of 73.8
percent (24.6). The maximum average (95% C.I.) was in General Activity
(21.5/25) (20.60-22.40) and the minimum in Nutrition (20/25)
(18.95-21.05), Physical examination scored an average of (20.7/25)
(19.6-21.6).
The spirometry findings were as follows: mean FEV1%
predicted (S.D.) was 62.7% (24.7%), mean FVC% predicted (S.D.) was at
64.2% (24.3%) and mean PEF % predicted (S.D.) was 61.6% (26.6%). The
mean CFQ-R scores in different domains across the different versions are
shown in Table I. The mean HRQoL score (95% C.I.) was
71.5/100 (68.51-74.63) for the Child version, 65.8/100 (54.64-77.07) for
the Adolescents and was 63 (59.24-66.81) according to the proxy rating
by parents.
TABLE I HRQoL Scores Domain wise {6-13 years (n=45), 14 years and above (n=14) and Parents (n=45)}
|
Domains/ |
No. of |
Mean scores |
Cronbach’s |
|
age group |
items |
(95% CI) |
alpha |
|
Physical |
|
6-13 |
6 |
65.7(57.37-73.96) |
0.863 |
|
≥ 14 |
8 |
56.4(41.77-70.94) |
0.722 |
|
Parents |
9 |
54.2 (47.73-60.56) |
0.786 |
|
Vitality |
|
6-13 |
– |
– |
– |
|
≥ 14 |
4 |
64.3 (50.8-77.8) |
0.657 |
|
Parents |
5 |
63.7 (57.6-69.8) |
0.734 |
|
Emotion |
|
6-13 |
8 |
78.8 (75.59-82.06) |
0.704 |
|
≥ 14 |
5 |
78.9 (65.72-92.14) |
0.817 |
|
Parents |
5 |
79.9 (74.95-84.78) |
0.441 |
|
Eat |
|
6-13 |
3 |
79.3 (72.75-85.79) |
0.440 |
|
≥ 14 |
2 |
77.4 (62.46-92.40) |
0.738 |
|
Parents |
2 |
73.3 (62.46-92.40) |
0.208 |
|
Treatment Burden |
|
6-13 |
3 |
62.1 (56.05-68.17) |
0.020 |
|
≥ 14 |
3 |
62.9 (53.95-71.91) |
0.026 |
|
Parents |
3 |
58.2 (51.23-65.06) |
0.347 |
|
Health Perceptions |
|
6-13 |
– |
– |
– |
|
≥14 |
3 |
73.1 (59.77-86.37) |
0.391 |
|
Parents |
3 |
61.4 (56.18-66.54) |
0.415 |
|
Social/ School |
|
6-13 |
7 |
63.7 (58.62-68.84) |
0.510 |
|
≥14 |
6 |
69.1 (56.00-82.29) |
0.362 |
|
Parents |
3 |
69.9 (63.33-76.42) |
0.119 |
|
Body |
|
6-13 |
3 |
65.3 (57.11-73.51) |
0.632 |
|
≥ 14 |
3 |
43.6 (22.02-65.27) |
0.797 |
|
Parents |
3 |
50.2 (43.59-56.82) |
0.559 |
|
Role |
|
6-13 |
– |
– |
– |
|
≥ 14 |
4 |
69.6 (55.53-83.61) |
0.766 |
|
Parents |
– |
– |
– |
|
Weight |
|
6-13 |
– |
– |
– |
|
≥14 |
1 |
47.6 (21.71-73.43) |
– |
|
Parents |
1 |
29.6 (19.12-40.14) |
– |
|
Respiratory |
|
6-13 |
4 |
73.5 (68.86-78.12) |
0.487 |
|
≥14 |
6 |
69.7 (53.39-86.04) |
0.857 |
|
Parents |
6 |
68.7 (64.61-72.70) |
0.620 |
|
Digestion |
|
6-13 |
1 |
84.6 (79.60-89.60) |
– |
|
≥14 |
3 |
77.9 (63.37-92.35) |
0.672 |
|
Parents |
3 |
84.5 (80.65-88.24) |
0.148 |
|
HRQoL score (%) |
|
6-13 |
35 |
71.6 (68.51-74.63) |
– |
|
≥14 |
50 |
65.9 (54.64-77.07) |
– |
|
Parents |
44 |
63.0 (59.24-66.81) |
– |
Reliability: For the child version, the
Cronbach’s alpha values were calculated for 7 domains (Digestion
excluded because of only 1 test item), the domains Physical (0.86),
Emotional (0.70), Body Image (0.63) were acceptable. Social
domain was intermediate (0.532). The domains Eating, Treatment Burden
and Respiration were unacceptable. Deleting question 33 from the
Respiration domain would elevate the alpha value to 0.58 making it
acceptable. For Adolescent version, reliability analysis showed that
among 11 domains tested (Weight excluded because of single test item),
all domains showed good reliability; Physical (0.722), Vitality
(0.65), Emotion (0.8), Eat (0.73), Body (0.79),
Role (0.76), Respiration (0.85), Digestion (0.67), except
for Treatment Burden, Health Perceptions and Social domains. Reliability
analysis of proxy rating by parents showed mixed results with Physical
(0.78), Vitality (0.73) and Respiration (0.62) faring
well; Body (0.56) barely acceptable and Emotional, Eat, Treatment
Burden, Health Perceptions, School, Digestion performing poorly.
Deleting question 31 may make Treatment Burden reliable.
Construct validity: For the 6-13 questionnaire
format, all the items of the Physical factor are assigned to the
Physical factor according to Factor analysis but the rest differ. The
English CFQ-R has 8 domains but the Factor analysis of our study
recommends a maximum of 5 factors (Web Fig. 1a). For the
Adolescent’s format, the English CFQ-R has 12 domains but Factor
analysis suggests a maximum of 7 factors (Web Fig 1b).
None of the items are allocated to a single factor. In the Parents’
format most of the items of the Physical factor are assigned to the
Physical factor according to Factor analysis but the rest differ. The
English CFQ-R has 11 domains but Factor analysis recommends a maximum of
2 factors (Web Fig 1c).
Known-group validity: We did not find any
difference in self-rated HRQoL scores in any domain between girls and
boys for all age groups. However, a statistically significant difference
was found with girls scoring higher than boys in proxy-rated HRQoL
scores for Physical (P= 0.03) and Treatment burden
(P=0.02) domains. A higher self-rated mean (SD) HRQoL scores in
Respiratory domain for Pseudomonas spp negative [76.9 (14.8)] as
compared to Pseudomonas spp positive for subjects in the age
group 6-13 years [67.2 (15.1)] (P=0.042). No such statistically
significant difference was found in HRQoL scores between culture
negative and culture positive subjects aged 14 and above and for proxy
rating. We found better mean (SD) self-rated HRQoL scores in Respiration
domain for groups with higher FEV1 values, i.e., FEV1% pred>80%
[82.5 (9.7)] compared to 40-59% [63.6 (19.1)] in subjects of ages 6-13
years (P=0.01). Similar analysis of HRQol scores obtained by proxy
rating by parents showed higher scores in Health perceptions (P=0.006)
and Respiration (P=0.005) domains and for the FEV1% predicted
>80% compared to <40% (P=<0.05). Similar differences were found
with higher scores in PEFR percent predicted group >80% compared to <40%
in proxy rated HRQoL scores in domain respiration. No difference were
found in self rated HRQoL scores for any of spirometric parameters for
age group 14 and above.
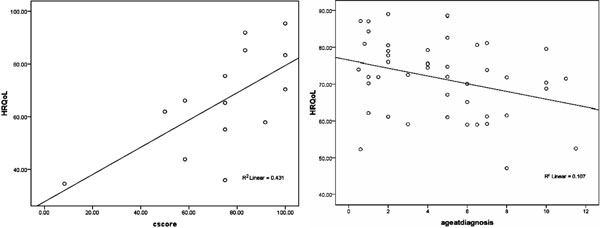
Correlation: There was a statistically
significant correlation between self-rated HRQoL scores and Clinical
scores for subjects aged 14 and above (r=0.65, P=0.011) (Fig
1a). No such correlation was found for age 6-13 proxy rating by
parents. There was a statistically significant inverse correlation
between self-rated HRQoL scores and age at diagnosis for ages 6-13;
(r=-0.339, P=0.02) (Fig. 1b). No such correlation
was found for age 14 and above, proxy rating by parents.
 |
|
Web Fig. 1 Scree Plot (a) 6-13
years format (b) Adolescent’s format (c) Parents’ format.
The point at which the plot sharply dips is the maximum number
of factors recommended. Our analysis suggests reduction of the
number of domains for Hindi CFQ-R to 5, 7 and 2 for 6-13,
Adolescent’s and Parents’ formats, respectively.
|
Convergent validity: Reliability analysis showed
statistically significant correlation between self and proxy rating for
4 out of 8 domains; Physical (ICC=0.36, P= 0.008), Eat (ICC=0.42,
P=0.002), Respiration (ICC=0.58, P=0.0001), Digestion
(ICC=0.37, P= 0.006). The other domains did not show any
statistically significant correlation.
Discussion
This is the first study looking at HRQoL of patients
diagnosed with cystic fibrosis in a Hindi-speaking population.
Our study demonstrated good internal consistency in most domains,
especially in the adolescent version. We also noted a pattern of domains
with less number of items (viz. Treatment Burden, Eating, Digestion,
Health Perceptions, School) having poor internal consistency across all
versions.
Our study trends the quality of life through
the different life stages. It also combines the self–rating with an
independent proxy rating by the parents. The low to moderate correlation
observed in many studies establishes the importance of both the ratings
to get a holistic picture of the quality of life of children with CF
[27]. Moreover, the observed correlations with objective health
parameters renders a new clinical dimension to the questionnaire,
proving that it is sensitive enough to detect clinically significant
changes. Our study has a few limitations too. Firstly, in a
cross-sectional study, design-causation cannot be proved; and secondly,
this being a single-center study, the sample size was not sufficient to
gain adequate power for the factor analysis.
The Principal Factor Analysis did not allocate most
of the items to the respective domains in the questionnaire, only items
belonging to the domain Physical had factor loadings >0.4 for the child
and the parents’ version. This may be due to the sample size,
considering inclusion of at least 10 subjects per item is recommended to
increase the power for Factor analysis [17]. The Factor
analysis also suggested reducing the number of domains across all the
versions, especially the parents’ version. This contrast with the good
Cronbach salpha values becomes difficult, especially in light of the
questionable importance of factor loadings for validation of a
psychometric tool involving causal items [24,25]. The mean scores were
greater for the self-rating in most domains, except for Emotion and
Social fshowing that the parents overestimated the emotional and social
quality of life of their children.
The delayed diagnosis reported in this study is
possibly due to less awareness, and non-availability of sweat testing.
This contrasts with the statistics in areas where newborn screening is
in place [3]. This, however, has been found in earlier studies on cystic
fibrosis in India, attributed to lack of newborn screening and low
awareness of the existence of cystic fibrosis. We also found an inverse
correlation between age at diagnosis and the HRQoL scores for children
aged 6-13 years. To address the clinical relevance of the HRQoL scores,
we used objective clinical parameters and found significant
associations.
Our study replicated the low Cronbach’s alpha values
for domains Treatment Burden and Social, found in previous studies of
CFQ-R (17,18,21). The low correlation between the and proxy-rating in
this study has also been reported previously for Physical and Emotion
domains [17,18]. The domains Emotion, Treatment Burden, Social, Body
Image also showed no correlation, as seen in the Spanish study and the
study by Havermans, et al. [20,26]. The positive association
between scores in Respiration domain and spirometry [16] have also been
previously reported [16,26], thereby validating CFQ-14+ showing
correlation between pulmonary function and Respiration domain. Similar
to our study, an Italian study also found lower Respiration scores for
Pseudomonas positive, compared to negative patients [19].
We conclude that the Hindi version of CFQ-R is a
valid psychometric instrument. With a few changes in its present
structure and by addressing the present statistical shortcomings, it may
be possible to integrate CFQ-R Hindi in the clinics for monitoring the
QoL of cystic fibrosis patients.
Acknowledgements: We are thankful to Mr. Bharat
Bhushan Pandey for data entry.
Contributors: DK: Developed protocol, collected
data, analyzed data and wrote manuscript; SG: involved in data
collection and manuscript writing; GPJ involved in data collection; MK:
involved in data analysis and manuscript writing; RL: involved in
protocol development, and manuscript writing; SKK: Involved in protocol
development, data collection, data analysis and manuscript writing, will
act as guarantor for the paper.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Cystic fibrosis
specific English version of CFQ-R is a valid psychometric
instrument with profound clinical impact.
What This Study Adds?
• Cystic fibrosis specific Hindi version
of CFQ-R is a valid psychometric instrument.
|
References
1. Kabra SK, Kabra M, Lodha R, Shastri S. Cystic
fibrosis in India. Pediatr Pulmonol. 2007;42:1087-94.
2. Kabra SK, Kabra M, Lodha R, Shastri S, Ghosh M,
Pandey RM, et al. Clinical profile and frequency of deltaf508
mutation in Indian children with cystic fibrosis. Indian Pediatr.
2003;40:612-9.
3. CF Foundation Patient Registry Annual Data Report
2012. Available from:
www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf.
Accessed August 15, 2015.
4. Staab D, Wenninger K, Gebert N, Rupprath K, Bisson
S, Trettin M, et al. Quality of life in patients with cystic
fibrosis and their parents: what is important besides disease severity?
Thorax. 1998;53:727-31.
5. Cella DF. Quality of life: the concept. J Palliat
Care. 1992;8:8-13.
6. Gee L, Abbott J, Conway SP, Etherington C, Webb
AK. Validation of the SF-36 for the assessment of quality of life in
adolescents and adults with cystic fibrosis. J Cyst Fibros.
2002;1:137-45.
7. Gee L, Abbott J, Conway SP, Etherington C, Webb
AK. Development of a disease specific health related quality of life
measure for adults and adolescents with cystic fibrosis. Thorax.
2000;55:946-54.
8. Abbott J, Hart A. Measuring and reporting quality
of life outcomes in clinical trials in cystic fibrosis: a critical
review. Health Quality Life Outcomes. 2005;3:19-22.
9. Center for Drug Evaluation and Research Guidance
for industry. Patient-reported outcome measures: Use in medical product
development to support labeling claims. US Department of Health and
Human Services, Food and Drug Administration. December 2009. Available
from: www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf.
Accessed August 15, 2014.
10. Goss CH, Quittner AL. Patient-reported outcomes
in cystic fibrosis. Proceedings of the American Thoracic Society.
2007;4:378-86.
11. Quittner AL. Measurement of quality of life in
cystic fibrosis. Curr Opin Pulm Med. 1998;4:326-31.
12. Henry B, Aussage P, Grosskopf C, Goehrs JM.
Development of the Cystic Fibrosis Questionnaire (CFQ) for assessing
quality of life in pediatric and adult patients. Qual Life Res.
2003;12:63-76.
13. Quittner AL, Sweeny S, Watrous M, Munzenberger p,
Bearss K, Gibson Nitza A, et al. Translation and linguistic
validation of a disease-specific quality of life measure for cystic
fibrosis. J Pediatr Psychol. 2000;25:403-14.
14. Modi AC, Quittner AL. Validation of a
Disease-specific measure of health-related quality of life for children
with cystic fibrosis. J Pediatr Psychol. 2003;28:535-46.
15. Quittner AL, Buu A, Messer MA, Modi AC, Watrous
M. Development and validation of The Cystic Fibrosis Questionnaire in
the United States: a health-related quality-of-life measure for cystic
fibrosis. Chest. 2005;128: 2347-54.
16. Klijn PH, van Stel HF, Quittner AL, van der Net
J, Doeleman W, van der Schans CP, et al. Validation of the Dutch
cystic fibrosis questionnaire (CFQ) in adolescents and adults. J Cyst
Fibros. 2004;3:29-36.
17. Schmidt A,Wenninger K,Niemann N, Wahn U,Staab D:
Health-related quality of life in children with cystic fibrosis:
validation of the German CFQ-R. Health Quality Life Outcomes.
2009;7:97-106.
18. Quittner AL, Sawicki GS, McMullen A, Rasouliyan
L, Pasta DJ, Yegin A, et al. Psychometric evaluation of the
Cystic Fibrosis Questionnaire-Revised in a national sample. Qual Life
Res. 2012;21:1279-90.
19. Bodnar R, Kadar L, Holics K, Ujhelyi R, Kovacs L,
Bolbas K, et al. Factros influencing quality of life and disease
severity in Hungarian children and young adults wth cystic fibrosis.
Ital J Pediatr. 2014;40:50.
20. Groeneveld IF, Sosa ES, Pe´rez M, Fiuza-Luces C,
Gonzalez-Saiz L, Gallardo C, et al. Health-related quality of
life of Spanish children with cystic fibrosis. Qual Life Res.
2012;21:1837-45.
21. Yuksel H, Yilmaz O, Dogru D, Karadag B, Unal F,
Quittner AL. Reliability and validity of the Cystic Fibrosis
Questionnaire-Revised for children and parents in Turkey:
cross-sectional study. Qual Life Res. 2013;22:409-14.
22. Sharma VK, Raj D, Xess I, Lodha R, Kabra SK.
Prevalence and risk factors for allergic bronchopulmonary aspergillosis
in Indian children with cystic fibrosis. Indian Pediatr. 2014;51:295-7.
23. Peterson RA, Kim Y. On the relationship between
coefficient alpha and composite reliability. J Appl Psychol.
2013;98:194-8.
24. Fayers PM, Hand DJ. Factor analysis, causal
indicators and quality of life. Qual Life Res. 1997;6:139-50.
25. Juniper EF, Guyatt GH, Streiner DL, King DR.
Clinical impact versus factor analysis for quality of life questionnaire
construction. J Clin Epidemiol. 1997;50:233-8.
26. Havermans T, Vreys M, Proesmans M, De Boeck C.
Assessment of agreement between parents and children on health-related
quality of life in children with cystic fibrosis. Child Care Health Dev.
2006;32:1-7.
27. Janse AJ, Sinnema G, Uiterwaal CS, Kimpen JL,
Gemke RJ. Quality of life in chronic illness: children, parents and
paediatricians have different, but stable perceptions. Acta Paediatr.
2008;97:1118-24.
|
|
|
 |
|

