|
|
|
Indian Pediatr 2013;50: 501-503 |
 |
Plasma Endothelial Microparticles, TNF- α
and IL-6 in Kawasaki Disease
|
|
Zhen Tan, *Yan Yuan, Sun Chen, Yi Chen and
#Tong-Xin
Chen
From the Department of Pediatrics, Xinhua Hospital
affiliated to Shanghai Jiao Tong University School of Medicine, China,
200092; *Department of Public Health Sciences, University of Alberta,
Canada T6G IC9; #Department of Clinical Immunology/Department of
Nephrology and Rheumatology, Children’s Hospital of Shanghai, Shanghai
Jiao Tong University, Shanghai, China, 200040.
Correspondence to: Dr Tong-Xin Chen, Department of
Clinical Immunology/Department of Nephrology and Rheumatology,
Children’s Hospital of Shanghai, Shanghai Jiao Tong University, No. 24,
Lane 1400, Western Beijing Road, Shanghai 200040, China.
Email: [email protected]
Received: September 09, 2011;
Initial review: October 10, 2011;
Accepted: October 22, 2012.
PII: S097475591100751
|
We studied the levels of endothelial
microparticles (EMPs), IL-6, and TNF-
a
in patients with Kawasaki disease (KD). EMPs were enumerated by flow
cytometry, while IL-6 and TNF-a
were measured using enzyme-linked immunosorbent assay. EMPs and IL-6
were elevated in KD, the level of TNF-a
in KD was not different from disease controls, but higher than
healthy controls. EMPs were positively correlated with TNF-a
and negatively correlated with albumin. Elevated level of EMPs, a
biomarker of endothelial cells damage, concomitant with increased
levels of TNF-a
and IL-6, is seen in patients with KD.
Key words: Endothelial microparticles; Interleukin-6;
Kawasaki disease; Tumor necrosis factor- a.
|
|
K awasaki disease (KD) is an
acute febrile autoimmune vasculitis with unclear etiology. Its diagnosis
is based on symptoms without specific
diagnostic test. A delay in diagnosis could result in a
delay in treatment, which is associated with a 25% probability of
coronary artery lesions [1]. Therefore, an early diagnosis of KD is
clinically important.
Endothelial microparticles (EMPs), budded from
endothelial cells, have been suggested as a possible marker of
endothelial disturbance [2]. Elevated EMPs were detected in patients
from a number of vascular disorders, e.g. systemic vasculitis [3]. Few
studies have assessed these particles in children with KD.
Methods
Patients with KD under 36-month of age who met the
diagnostic criteria [4] within 10 days of the onset of fever, were
enrolled between March 2009 and April 2010. Age and sex matched patients
with acute infectious febrile disease and healthy children were enrolled
as disease controls and healthy controls, respectively. Following
institutional ethics approval and informed parental consent, blood
samples were collected from all subjects before therapy with intravenous
immunoglobulin for KD group.
Clinical symptoms were obtained from observations and
interviews of the primary caregivers. Laboratory results including white
blood cell count, absolute neutrophil count, platelet count, erythrocyte
sedimentation rate, C-reactive protein and albumin were obtained.
Citrated venous blood (1 mL) was centrifuged
immediately twice for 5 minutes at 5000g at room temperature to obtain
platelet poor plasma, which was stored at -80°C. A 50 µL aliquot was
incubated with 10 µL of FITC labeled anti-CD31 BD Biosciences, Franklin
Lakes, NJ, USA) and 10 µL of phycoerythrin labeled anti-CD146 (BD
Biosciences) at room temperature for 20 minutes in dark. Samples were
measured using Becton Dickinson FACSCalibur flow cytometry. The gate was
standardized by Megamix beads, forward scatter parameters were plotted
on logarithmic scales to <1.0 mm. All microparticles positive for CD31
and CD146 were counted as EMPs to a maximum value of 10,000.
Venous blood (2 mL) was collected in a gel
coagulation-promoting vacuum tube and centrifuged immediately for 15
minutes at 4000rpm at room temperature for serum, which was stored at
-80°C. IL-6 and TNF- a
in serum were measured by enzyme-linked immunosorbent assay (R&D,
Minneapolis, MN, USA) according to manufacturer’s instructions.
Statistical analysis: Sample size assessment and
power analysis was done by using OpenEpi (Version 2.3.1), with type I
error 5% and power of 80%. All results were expressed as mean±SD.
One-way analysis of variance (ANOVA) followed by Test of least
significant differences assuming equal variance or Tamhane’s T2 test
assuming unequal variances were used. Pearson correlation coefficients
were reported for EMPs and other variables of interest. The diagnostic
value of EMPs was assessed with receiver operating characteristic curves
and area under curve.
Results
We enrolled 20 KD patients, 18 disease controls and
20 healthy controls. 8 patients had atypical KD and one KD patient had
coronary lesions. KD and disease controls had recurrent fever; mean
duration of 6.1 and 8.1 days, respectively.
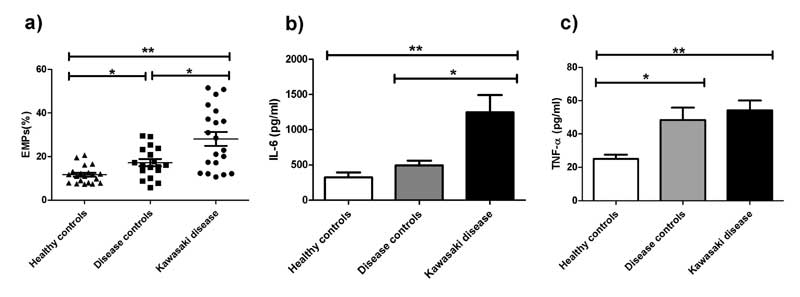
The percentage counts of EMPs were 28.07±14.16% in KD
children, which were significantly higher (P<0.001) than that of
disease controls (17.20±6.99%) and healthy controls (11.67±3.97%). The
respective serum concentrations of IL-6 were 1247.11±1093.02 pg/mL,
495.66±281.49 pg/mL and 326.08±302.69 pg/mL. The concentrations of TNF-α
were 54.32±25.59 pg/mL in KD children, 48.42±31.45 pg/mL in disease
controls and 25.12±11.0 pg/mL in healthy controls (Fig. 1).
EMPs were positively correlated with TNF-α
(P<0.001) and negatively correlated with albumin (P<0.001).
There were no significant correlations between EMPs and IL-6, white
blood cells and neutrophil count, C-reactive protein, erythrocyte
sedimentation rate or platelet counts.
 |
| |
As shown in
Web Fig. 1, the area under
curve for predicting KD using EMPs among febrile patients (KD and
disease controls) was 0.714. When a cutoff value of 20.99% was chosen
based on highest Youden index of receiver operating characteristic
curves for predicting KD, it generated a specificity of 0.72 and
sensitivity of 0.60.
Discussion
Microparticles are vesicles consisting of variable
amounts of cytoplasmic components and surface phospholipids when
compared to their parental cells [5]. In non-disease states, the release
of microparticles is programmed, and increased microparticles levels are
triggered by activation of apoptosis or cell lysis [6]. EMPs,
microparticles from endothelial cells, have a varied group of surface
biomarkers, such as CD31+, CD42b-, CD62E+, CD144+ or CD146+/CD105-. The
combination of multiple biomarkers is a more specific assessment of EMPs
due to the co-expression of these markers on the other cells or
platelets. We choose CD31+/CD146+ to characterize EMPs [7,8]. The level
of EMPs in KD patients was significantly higher than that of disease
controls and healthy controls, and was negatively correlated with serum
albumin. Since the decreased serum albumin represents the vascular
leakage by the damage to the vascular endothelium, the results confirmed
that the level of CD31+/CD146+ EMPs is a potential biomarker of
endothelial cell damage and the damage starts in the early stage of KD.
However, a relatively low sensitivity of 0.60 in ROC curve suggested
that EMPs alone was not highly sensitive.
IL-6 and TNF- a
are potent pro-inflammatory cytokines and have multiple immune-modulatory
functions. High levels of IL-6 could inhibit the differentiation of Th1
cells while promoting Th2 cells activation and consequently increasing
the production of Th2 cytokines. Subsequently, they activate the
polyclonal B cells to produce autoantibody in patients [9]. Therefore,
IL-6 is a critical cytokine in the KD pathogenesis of autoimmune
vasculitis. The difference of serum IL-6 levels between KD and disease
controls may represent the fundamental difference of the two diseases.
In an animal model of KD induced by Lactobacillus
casei cell wall extract, the process of coronary arteritis and
aneurysms could be ablated by blocking TNF receptor [10], suggesting
that TNF- a is
capable of inducing coronary artery lesions directly. It has been
reported that TNF-a
activation could trigger the release of EMPs from endothelial cell in
vitro [11]. Consistently, we found that EMPs were correlated with
TNF-a but not
with IL-6. In our study, there were no differences in the levels of TNF-a
between the KD and disease controls. So far, it is agreed by most of
pediatricians that KD may be triggered by undefined infectious agents in
genetically predisposed individuals [12]. Therefore, the levels of TNF-a
were elevated in both KD and disease controls can be partly explained by
overlapping disease progression. However, since the levels of IL-6 and
EMPs were higher in KD than in disease controls one possible explanation
is that combination of TNF-a
with IL-6 will speed up the release of EMPs. An alternative is that some
unknown agents help TNF-a
to speed up the release of EMPs in KD.
To summarize, the level of EMPs, a potential
biomarker of endothelial cell damage, was elevated in KD patients and
was concomitant with high levels of IL-6 and TNF- a.
An increased level of IL-6 denotes a potential autoimmune response,
while elevated level of TNF-a
induces endothelial cell activation. The combination of these three
factors indicates that autoimmune vasculitis is fundamental in the
pathogenesis of KD.
Contributors: All the authors have contributed,
designed and approved the study.
Funding: None. Competing interests: None
stated.
|
What This Study Adds?
•
Endothelial microparticles are a biomarker of endothelial
cell damage in Kawasaki disease.
|
References
1. Burns JC, Glode MP. Kawasaki syndrome. Lancet.
2004;364:533-44.
2. Jimenez JJ, Jy W, Mauro LM, Horstman LL, Bidot CJ,
Ahn YS. Endothelial microparticles (EMP) as vascular disease markers.
Adv Clin Chem. 2005;39:131-57.
3. Clarke LA, Hong Y, Eleftheriou D, Shah V, Arrigoni
F, Klein NJ, et al. Endothelial injury and repair in systemic
vasculitis of the young. Arthritis Rheum. 2010;62: 1770-80.
4. Newburger JW, Takahashi M, Gerber MA, Gewitz MH,
Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term
management of Kawasaki disease: a statement for health professionals
from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki
Disease, Council on Cardiovascular Disease in the Young, American Heart
Association. Pediatrics. 2004;114:1708-33.
5. Enjeti AK, Lincz LF, Seldon M. Detection and
measurement of microparticles: an evolving research tool for vascular
biology. Semin Thromb Hemost. 2007;33:771-9.
6. Enjeti AK, Lincz LF, Seldon M. Microparticles in
health and disease. Semin Thromb Hemost. 2008;34:683-91.
7. Widemann A, Sabatier F, Arnaud L, Bonello L, Al-Massarani
G, Paganelli F, et al. CD146-based immunomagnetic enrichment
followed by multiparameter flow cytometry: a new approach to counting
circulating endothelial cells. J Thromb Haemost. 2008;6:869-76.
8. Harrison M, Murphy RP, O’Connor PL, O’Gorman DJ,
McCaffrey N, Cummins PM, et al. The endothelial microparticle
response to a high fat meal is not attenuated by prior exercise. Eur J
Appl Physiol. 2009;106:555-62.
9. Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang
KD. Serum albumin level predicts initial intravenous immunoglobulin
treatment failure in Kawasaki disease. Acta Paediatr. 2010;99:1578-83.
10. Chang J, Lu JR, Liang D. The function of Th1/Th2
cells in children with acute Kawasaki disease. Zhonghua Er Ke Za Zhi.
2006;44:377-8.
11. Sofi MH, Li W, Kaplan MH, Chang CH. Elevated IL-6
expression in CD4 T cells via PKCtheta and NF-kappaB induces Th2
cytokine production. Mol Immunol. 2009;46:1443-50.
12. Galeotti C, Bayry J, Kone-Paut I, Kaveri SV.
Kawasaki disease: aetiopathogenesis and therapeutic utility of
intravenous immunoglobulin. Autoimm Rev. 2010;9:441-8.
|
|
|
 |
|

