|
|
|
Indian Pediatr 2013;50: 473-476
|
 |
Effect of Second Dose of Measles Vaccine on
Measles Antibody Status:
A Randomized Controlled Trial
|
|
Anjum Fazilli, Abid Ali Mir, Rohul Jabeen Shah, Imtiyaz Ali
Bhat, *Bashir Ahmad Fomda and
†Mushtaq Ahmad Bhat
From the Departments of Community Medicine,
*Microbiology and †Pediatrics, Sher-i-Kashmir Institute of
Medical Sciences, Soura, Srinagar, J&K, India.
Correspondence to: Dr Mushtaq Ahmad Bhat,
Additional Professor Pediatrics, SKIMS, Srinagar, Kashmir,
India.
Email:
[email protected]
Received: July 12, 2012;
Initial review: September 20, 2012;
Accepted: October 25, 2012.
|
Objective: To evaluate the effect of the second dose of measles
vaccine on measles antibody status during childhood.
Setting: Immunization centre of Under-five Clinic
of the Department of Community Medicine at a tertiary-hospital.
Design: Randomized Controlled trial.
Methods: Blood samples were collected from all
subjects for baseline measles serology by heel puncture at 9-12 months
of age. All subjects were given the first dose of measels vaccine. At
second visit (3-5 months later), after collecting the blood sample from
all, half the children were randomized to receive the second dose of
measles vaccine (study group), followed by collection of the third
sample six weeks later in all the subjects.
Results: A total of 78 children were enrolled and
30 children in each group could be analyzed. 11(36.6%) children in the
study group and 13 (43.3%) children in the control group had protective
levels of measles IgG at baseline. Around 93.3 % of children in the
study group had protective measles antibody titers as against 50% in the
control group at the end of the trial. The Geometric Mean Titre (GMT) of
measles IgG increased from 14.8 NTU/mL to 18.2 NTU/mL from baseline to
six weeks following receipt of the second dose of the vaccine in the
study group, as compared to a decrease from 16.8 NTU/mL to 12.8 NTU/mL
in the control group.
Conclusions: A second dose of measles vaccine
boosts the measles antibody status in the study population as compared
to those who receive only a single dose.
Key words: Immunization, India, Measles, Prevention,
Second-dose, Serology.
|
|
Measles is a leading cause
of death among young children. Many experts now
recommend two doses of the measles vaccine to
ensure immunity, as about 15% of vaccinated
children fail to develop immunity from a single
dose [1]. In the past, there was a concern that
early immunization of infants who still have the
maternal antibody modified the immune response
such that the infant would not respond
adequately to a second dose. However, most
studies have shown that the overall proportion
of children who are seropositive after primary
immunization before 12 months of age and
re-immunized at age 15 months or later is at
least 95%, similar to that after initial
immunization at 15 months. Epidemiological data
support the efficacy of a second dose in the
presence of maternal antibody. Many countries
have initiated a two dose Measles, Mumps and
Rubella vaccine schedule with the aim of
eliminating Rubella and Measles [2]. The
rationale for the second dose has been to
protect those who did not seroconvert after the
first dose of measles vaccine. In an outbreak
investigation in USA, attack rates were 30-60%
lower in persons who received two doses of
measles vaccine as compared to those who
received one dose only [3].
In India, one dose of measles
vaccine is given under Universal Immunization
Program at 9 months of age. As some developing
countries have adopted a 2-dose schedule, Indian
Academy of Pediatrics has now recommended second
dose of measles vaccine at 15 months of age.
There is paucity of literature regarding the
effect of second dose of measles vaccination on
serological status in developing countries,
especially in the Indian subcontinent. Hence the
present study was designed to evaluate the
effect of second dose of measles vaccine on
measles antibody status during childhood.
Methods
This was a randomized
controlled trial conducted in the Under-five
Immunization Clinic of Department of Community
Medicine at Sher-i-Kashmir Institute of Medical
Sciences, Srinagar, Kashmir; a tertiary-care
hospital in northern India. The study was
conducted from May, 2008 to February, 2009. The
required sample size for the study was
calculated to be 30 in each arm [4]. A total of
78 subjects were enrolled in the study giving
allowance for the attrition factor. The study
was approved by the Ethical Committee of the
Institute.
Every alternate infant (age
9-12 months) reporting for measles vaccination
on the given day was enrolled in the study.
These infant were divided into two groups, one
designated as study group that received second
dose of measles vaccine 3-5 months after the
first dose at 9-12 months of age and the other
as control group receiving similar dose of
placebo (normal saline) after 1 st
dose of measles vaccine at 9-12 months of age.
Since measles vaccination is done only once in a
week, the average children vaccinated at the
immunization center on each week’s immunization
day was 20-25 and the process of enrollment of
the infants was completed in around eight
sessions.
Infants with a history of
measles, infants with a history of measles in
the family or immediate neighborhood during the
past one month, and infants whose age could not
be ascertained were excluded. Written consent
was obtained from the parents/guardians of
participants on a form that provided relevant
details of the study.
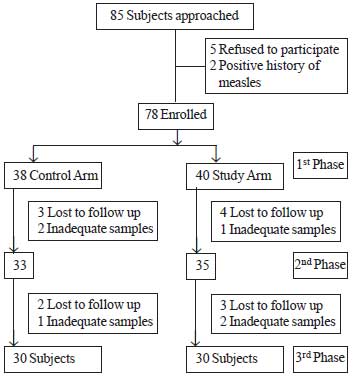 |
|
Flow chart showing
flow of infants in the study.
|
Pertinent information was
collected from the mother and entered into a
pre-designed proforma. The data information
related to demography, breastfeeding, weaning,
immunization and disease history. Subjects were
examined, their weight and height, head
circumference, and mid-upper arm circumference,
was measured. Weight was assessed using pan type
weighing scale. Height was measured using an
infantometer. Head circumference and Mid -arm
circumference was assessed using an inch tape.
Three blood samples were
collected from each patient and were processed
as per the manufacturer’s recommendations and
results were expressed in Nova Tech units [5,6].
Chi square test and Fisher test were used for
analysis.
Results
A total of 60 children were
enrolled in the study, 30 for the study group
and 30 for the control group. At enrollment, the
baseline variables of children in two groups
were comparable (Table I).
The proportion of children with protective
levels of measles IgG and the geometric mean
titers (GMT) of measles IgG were almost similar
in both groups at baseline. Measles IgG for
study group was similar between the two groups,
but it was significantly higher in the study
group at third visit (Table II).
TABLE I Demographic Details of The Study Population (N=60)
|
Study group |
Control group |
|
Females (%) |
8 (26) |
11 (36) |
|
Age |
|
|
|
First phase |
9.5 (0.73) |
9.53 (0.68) |
|
2nd phase |
13.8 (1.33) |
13.4 (1.24) |
|
Weight (kg) Mean ± SD |
|
|
|
First phase |
8.08 (1.01) |
8.14 (1.03) |
|
2nd phase |
9.74 (1.37) |
9.49 (1.11) |
|
Height (cm), Mean ± SD |
|
|
|
First phase |
68.9 (4.38) |
70.3 (4.92) |
|
2nd phase |
74.8 (5.16) |
74.5 (4.34) |
|
Literate mothers (%) |
9 (30) |
17 (56.6) |
|
Working mothers (%) |
1 (3.3) |
2 (6.7) |
TABLE II Measles IgG Titers in Studied Children During Three Phases of the Trial (N=60)
|
Study Group |
Control Group |
P |
|
n=30(%) |
n=30(%) |
value |
|
First phase |
|
|
|
|
No. with protected titers |
11(36.6) |
13 (43.3) |
0.792 |
|
GMT (NTU/mL)* |
10.60 |
11.21# |
|
|
Second phase |
|
|
|
|
No. with protected titers |
23 (76.6) |
21 (70.0) |
0.771 |
|
GMT (NTU/mL)* |
14.93 |
12.11 |
|
|
Third phase |
|
|
|
|
No. with protected titers |
28 (93.3) |
15 (50.0) |
0.0004 |
|
GMT (NTU/mL)* |
18.19 |
9.04 |
|
The GMT of measles IgG when
compared between protected and non-protected
children across two groups was higher for study
group than control group, both at the second and
third visit, though the difference was not
statically significant (Table III).
TABLE III Comparison Between GMJT of (NTU/mL) Measles IgG of the Study and Control Group
|
Study Group |
Control Group |
P |
|
n=30(%) |
n=30(%) |
value |
|
First phase |
|
Protected |
14.84 |
16.86 |
0.880 |
|
Not protected |
8.78 |
8.52 |
|
|
Second phase |
|
Protected |
16.91 |
13.12 |
0.643 |
|
Not protected |
9.38 |
8.36 |
|
|
Third phase |
|
Protected |
18.49 |
12.8 |
0.492 |
|
Not protected |
10.13 |
8.97 |
|
Discussion
The study found that a second
dose of measles vaccine boosts IgG levels
post-vaccination, as compared to children
receiving one dose approximately 3-5 months
earlier.
The choice of the serological
assay is important in evaluating the response to
immunization. Both Plaque Nutralization assays
and ELISA are more sensitive than the
Heamagglutination Inhibition assays [6]. At
present no serological test can differentiate
between antibodies (whether IgG or IgM) produced
by measles infection and that produced by
immunization. The levels of antibody induced by
immunization with attenuated measles virus vary
with an approximately log-normal distribution.
The proportion of children
having protective levels of measles IgG at 1 st
visit in this study was higher than 3.5%- 17.6%
reported in the literature [7-9]. The reason for
our observation could be a pre-vaccination
exposure to wild measles virus infection since
measles is highly endemic in this region.
At the third visit, which was
scheduled at six weeks following the receipt of
second dose of measles vaccine in study group
and a placebo in control group, a significantly
higher number of children had protective levels
of measles IgG in the study group. Similar
results (93.7% vs 84.7%, respectively)
have been reported from North Korea [10].
However, in our study, the proportion of
protected children in the control group
decreased from 70% to 50% from second to third
visit. The explanation for this observation
could be that since the proportion of children
who had protective levels of measles IgG
antibodies at base line was quite high (43.3%),
which could either have been due to maternal
antibodies or natural infection. Measles being
highly endemic in this region, thus the
proportion that could have responded to measles
vaccine was actually less and the group which
did not respond to measles vaccine at nine
months continued with the waning phenomenon.
The GMT of IgG rose by 14.2 %
from first visit to second visit and by 8.8 %
from second to third visit in the study group,
while it gradually decreased in the control
group. An attempt was made to compare the
nutritional status of the children and immune
status but a significant relation could not be
established.
Our study proves that second
dose of measles vaccine boosts the measles IgG
status in the study population as compared to
those who received only single dose. We also
observed that in the control group the
proportion of one dose vaccinated children
initially increased and then returned to almost
the same proportion protected at pre-vaccination
levels. This justifies the need for a second
dose of measles vaccine.
Contributors: All the
authors have designed and approved the
manuscript.
Funding: Research
Grant from Academic Section SKIMS; Competing
interests: None stated.
References
1. World Health Organization.
Measles. Available from:
www.who.int/mediacentre/fact sheet/fs286/en/.
Accessed on 2 October, 2012.
2. Measles –Immunological
basis for immunization /module 7: measles
WHO/EPI/GEN/93.7 Page-4. Accessed on 2 October,
2012.
3. Vitek CR, Aduddell
M, Brinton MJ, Hoffman RE, Redd SC. Increased
protections during a measles outbreak of
children previously vaccinated with a second
dose of measles-mumps-rubella vaccine. Pediatr
Infect Dis J. 1999;18:620-3.
4. Kirby A, Gebski V, Keech
AC. Determining the sample size in a clinical
trial. Med J Aust. 2002;177:256-7.
5. Riddell MA, Leydon JA,
Catton MG, Kelly HA. Detection of measles
virus-specific immunoglobulin M in dried venous
blood samples by using a commercial enzyme
immunoassay. J Clin Microbiol. 2002:40:5-9.
6. Nakano JH, Miller DL,
Foster SO, Brink EW. Microtiter determination of
measles haemagglutination inhibition antibody
with filter papers. J Clin Microbiol. 1983;17:
860-3.
7. Khalil MK, Nadrah HM, Al-Yahya
OA, Al-Saigul AM. Sero-response to measles
vaccination at 12 months of age in Saudi infants
in Qassim province. Saudi Med J.
2008;29:1009-13.
8. Mandomando IM, Naniche D,
Pasetti MF, Vallies X, Cuberos L, Nhacola A,
et al. Measles specific neutralizing
antibodies in rural Mozambique: sero-prevalence
and presence in breast milk. Am J Trop Med
Hyg.2008; 79: 787-92.
9. Jani JV, Holm-Hansen C,
Mussa T, Zaqngo A, Manhica I, Bjune G, et al.
Assessment of measles immunity among infants in
Maputo city, Mozambique. BMC Public
Health. 2008;8:386.
10. Bae GR, Lim HS, Goh UY,
Yang BG, Kim YT, Lee JK. Seroprevelence of
measles antibody and its attributable factor in
elementary students of routine 2- dose schedule
era with vaccination record. J Prev Med Public
Health. 2005;38:431-6.
|
|
|
 |
|

