|
|
|
Indian Pediatr 2013;50:
459-462 |
 |
Serum Neutrophil Gelatinase-Associated
Lipocalin as a Marker of Acute Kidney Injury in Asphyxiated
Neonates
|
|
NM El Raggal, SM Khafagy, *NH Mahmoud and SA El Beltagy
From the Departments of Pediatrics, and *Clinical
Pathology, Faculty of Medicine - Ain Shams University, Egypt.
Correspondance to: Dr. Soha Mohamed
Khafagy, Pediatric Department, Ain Shams University, Abbassayia, Cairo,
Egypt. Email:
[email protected]
Received: July 23, 2012;
Initial review: August 13, 2012;
Accepted: October 09, 2012 .
PII:S097475591200659
|
Objective: To determine the clinical utility of serum neutrophil
gelatinase-associated lipocalin (NGAL) as an early marker of acute
kidney injury in asphyxiated neonates with hypoxic ischemic
encephalopathy (HIE).
Design: Cohort study.
Settings: National Intensive Care Unit of
Maternity Hospital, Ain Shams University, Cairo, Egypt.
Patients: The study included 30 term asphyxiated
neonates (8 with mild, 13 with moderate and 9 with severe HIE) and 20
control neonates.
Intervention: Serum NGAL level was measured
within 6 hours after birth using an enzyme linked immunosorbent assay.
Main outcome measures: Patients were subsequently
discriminated into AKI (n=12) and no-AKI (n=18) groups.
Results: The median (Interquartile range) serum
NGAL concentration was 95.0 (70.75-180.00) ng/mL in asphyxiated
neonates, and 39.75 (6.0-48.0) ng/mL in control neonates; (P<0.001).
Serum NGAL correlated with HIE severity: mean (SD) was 65.50 (3.77) ng/mL
in infants with mild HIE, 115.07 (45.83) ng/mL in infants with moderate
HIE and 229.66 (79.50) ng/mL in infants with severe HIE; (P<0.01).
The median (Interquartiles) serum NGAL level was 182.50 (166.25-301.75)
ng/mL in patients with AKI, 74.00 (66.00-78.75) ng/mL in those without
AKI; (P<0.001). A cutoff value 157 ng/mL for serum NGAL could
detect AKI in asphyxiated neonates with a sensitivity of 83.3% and a
specificity of 94.4%.
Conclusion: Elevated serum NGAL measured within 6
hours after birth reliably indicates acute kidney injury in asphyxiated
neonates.
Key words: Acute kidney injury, Asphyxia, Outcome.
|
|
A
cute kidney injury (AKI) is a common consequence
of perinatal asphyxia [1] occurring in up to 56% of these infants
[2]. The mechanisms of AKI in asphyxiated neonates include diminished
renal blood flow because of hypovolemia and hypotension, which can lead
to impaired GFR and tubular function [3,4]. In the newborn, the
diagnosis of AKI, particularly mild to moderate forms is difficult [5].
Detection of reduced kidney function with a rise in serum creatinine
concentration, is an unreliable measure in the acute setting [6]. So the
establishment of non-serum creatinine-based AKI diagnostic criteria is
crucial for this age group.
Neutrophil gelatinase-associated lipocalin (NGAL) is
a 25kDa secretory glycoprotein that belongs to the lipocalin family of
proteins. Human NGAL was originally isolated from the supernatant of
activated neutrophils. Renal expression of NGAL increases dramatically
after renal ischemia. This is reflected by the rapid rise in urinary
NGAL reported in AKI. NGAL concentration in the serum and urine has been
demonstrated to be a sensitive and specific early marker of AKI after
cardiac surgery [6,7].
This study was designed to assess serum NGAL level in
asphyxiated term neonates within 6 hours of birth, whenever urine
sampling is difficult, to evaluate its relation to HIE severity, and its
clinical utility for early detection of AKI in these neonates
Methods
This cohort study was conducted at National Intensive
Care Unit of Maternity Hospital of Ain Shams University, Cairo, Egypt,
over a period of 10 months from July 2008 till April 2009. The study was
approved by the Ethical Committee of the Pediatric Department at Ain
Shams University. An informed consent was obtained from one of parents
before enrollment of the patients.
Neonates included in the study were
³37 completed weeks
of gestation, appropriate for gestational age. Newborns with congenital
malformations, chromosomal abnormalities, suspected inborn error of
metabolism, sepsis; those born to diabetic or preeclamptic mothers;
outcomes of multiple gestations, and those born to mothers who received
nephrotoxic drugs were excluded from the study. They were divided into 2
groups:
(a) Patient Group: It included
30 term neonates with a provisional diagnosis of perinatal asphyxia
based on the criteria of American Academy of Pediatrics [8].
(b) Control Group: It included 20
apparently healthy neonates matched for gestational age, birthweight and
postnatal age.
Complete history was elicited from mothers including
maternal, obstetric, and perinatal history. Gestational age was
calculated based on the date of last menstrual period and confirmed by
neonatal examination using the modified Ballard score [9]. Birth weight,
sex, and Apgar score at 1, 5 and 10 minutes were recorded. Complete
physical examination was done with special emphasis on neurological
examination.
Laboratory investigations included complete blood
count, C-reactive protein, blood urea nitrogen, serum creatinine (done
daily for the first week of life) and serum NGAL (done within the first
6 hours of life), with daily assessment of urine output (24 hours
urine output measurement was done by applying plastic collection bag).
Oliguria was defined as urine output (<1 mL /kg/hour). Samples were
collected from the control group during sampling for bilirubin
measurement within the first 48 hours of life.
Patients were subsequently discriminated, following
48 hours of admission into AKI (n=12) and no-AKI (n=18)
groups. Acute kidney injury was defined as elevation of serum creatinine
>1.5 mg/dL for more than 48 hours [10]. Asphyxiated infants
were neurologically examined daily over the first
two postnatal weeks for the sequential appearance and resolution of
various transitory neurological signs and their duration and were
subsequently classified according to the Sarnat clinical stages [11] as
mild (grade I, n=8), moderate (grade II, n=13) and severe
(grade III, n=9) HIE.
Statistical analysis: Statistical analysis was
done using SPSS software package, version 15.0, (Ecosoft corporation,
USA). Data were expressed descriptively as mean ± standard deviation
(SD) for quantitative parametric data and median and interquartile range
for quantitative skewed data. Comparison between groups was done using
the student t test for parametric data and Wilcoxon’s rank sum
test for skewed data. Correlation study between the different analyzed
parameters was done using Spearman’s rank correlation coefficient test
for skewed data. The diagnostic performance of serum NGAL was evaluated
using receiver operating characteristic curve (ROC) analysis.
Results
The demographic and clinical characteristics and
laboratory data of studied neonates are listed in Table I.
Neonatal sepsis was excluded in all neonates of the study based on
clinical and laboratory criteria (normal blood cell counts, C- reactive
protein <6 mg/L and negative blood culture results). We demonstrated a
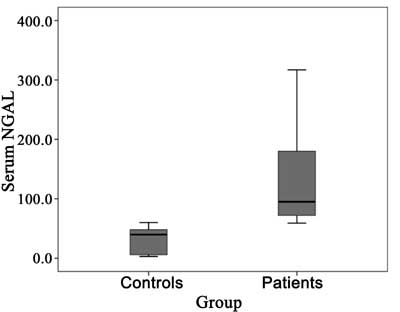
highly significant increase in sNGAL in patient group (median= 95.0 ng/mL,
IQ= 70.75-180.00) as compared with control group (median= 39.75 ng/mL,
IQ= 6.0-48.0) (P<0.001) (Fig. 1).
TABLE I Characteristics of The Study Children
|
Patient Group (n=30) |
Control Group (n=20) |
P value |
|
Gestational age (weeks) |
38.1 (1.29) |
38.1 (1.07) |
0.67 |
|
Birthweight (g) |
3250.0 (506.3) |
3317.5 (409.5) |
0.07 |
|
Apgar score 1 min
|
1.0 |
8.0 |
<0.001 |
|
5 min
|
3.0 |
8.0
|
<0.001 |
|
10 min |
6.0 |
10.0 |
<0.001 |
|
Male |
17(55) |
8 (40) |
0.72 |
|
Female |
13(45) |
12 (60) |
|
|
Vaginal delivery*
|
18(60) |
9 (45) |
0.72 |
|
Cesarean delivery* |
12(40) |
11 (55) |
|
|
Oliguria <1mL/kg/h (for the first 48 hours)* |
6 (20) |
– |
|
|
BUN (mg/dL) (within first 48 hours) Mean± SD |
39.20 (17.67) |
16.05 (7.74) |
< 0.001 |
|
Creatinine (mg/dL) (within first 48 hours)Mean ± SD |
1.73 (0.42) |
0.67 (0.22) |
< 0.001 |
|
BUN: blood urea nitrogen; All values in mean (SD); *No. (%). |
 |
|
Fig.1 Serum NGAL levels in patients and
control group.
|
Serum NGAL correlated with HIE severity. The mean
(SD) serum NGAL was 65.5 (3.77), 115.1 (45.83) and 229.7 (79.5) ng/mL in
no or mild HIE, moderate HIE and severe HIE groups, respectively. The
P values were <0.01 for all three comparisons viz. mild vs.
moderate HIE, moderate vs. severe HIE and mild vs. severe
HIE.
A significant positive correlation was found between
sNGAL and both serum creatinine and BUN levels determined after 48 hours
from birth (P < 0.05 and < 0.001, respectively). Patients who
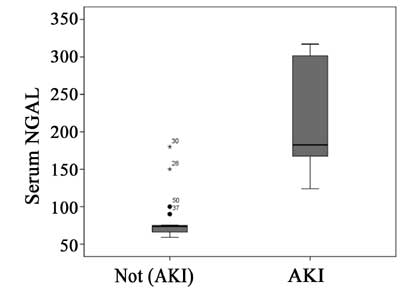
were subsequently diagnosed as having AKI, were found to have
significantly higher level of sNGAL (median=182.50, IQR=166.25-301.75 ng/mL)
compared with those without AKI (median=74.0, IQR=66.00-78.75 ng/mL) (P
< 0.001) (Fig. 2).
 |
|
Fig. 2 Serum NGAL level with and
without acute kidney injury.
|
Receiver operating characteristic curve had area
under the curve (AUC) of 0.968 with a confidence interval of (1.0-1.0);
P < 0.001. A serum NGAL cutoff value 157 ng/mL could
differentiate asphyxiated neonates with AKI from asphyxiated neonates
without AKI, with a sensitivity of 83.3%, specificity 94.4%,
positive predictive value 85.7% and negative predictive value 92.3% with
a diagnostic accuracy 90%.
Discussion
NGAL expression increases greatly in the presence of
inflammation and injured epithelia and therefore, NGAL is one of the
earliest proteins induced in the kidney after ischemic or nephrotoxic
insult. Consequently, NGAL significantly rises in blood and urine soon
after AKI [12].
In our study, serum NGAL measured in the first 6
hours of life showed significantly higher values in patient group than
control group. Serum levels of NGAL were also significantly higher in
cases with acute kidney injury than cases without AKI. This was in
agreement with another study done on asphyxiated neonates which found
that asphyxiated neonates had significantly higher serum NGAL and urine
NGAL (standardized to urine creatinine and absolute values) than
controls at days 1, 3,and 10 [13]. Similarly, the study done by
Krawczeski, et al. [6] observed that both plasma and urine NGAL
concentrations became markedly and significantly higher in both neonatal
and non neonatal patients with AKI.
Serum creatinine is not a good marker of renal
dysfunction in general and in the neonate there are specific problems
associated with it. First, the creatinine concentration reflects the
maternal level for up to 72 hours after birth, rendering it unhelpful in
the assessment of the neonate in the immediate postnatal period [14].
Second, large changes in the glomerular filtration rate (GFR) occur
in the absence of a change in serum creatinine. Moreover, there is
significant variability in neonatal GFR/ creatinine values, which change
rapidly in the immediate postnatal period as the infant adapts to
extrauterine life [15].
Renal failure in the neonate often occurs in the
absence of oliguria [3], and a high index of suspicion is required. We
depended mainly on serum creatinine levels because only 20% of our
studied cases had oliguria in the first day of life while 80% had normal
urine output. There was significant positive correlation between NGAL
and creatinine and BUN levels in cases group. This comes in agreement
with Bachorzewska-Gajewska, et al. [16] who found
that serum creatinine correlates significantly with both serum and
urinary NGAL. Furthermore, serum NGAL steadily increases across groups
when stratified according to RIFLE classification. Also in
a study done on patients with lupus nephritis, they found that NGAL
levels were strongly correlated with renal disease activity but not with
extrarenal disease activity score [17].
ROC analysis revealed that sNGAL at a cutoff value of
157 ng/mL, within the first 6 hours of life in asphyxiated neonates, can
predict the development of AKI with high sensitivity and specificity.
Similarly, another recently published study has demonstrated that serum
and urine NGAL could predict AKI in newborns experiencing acute
perinatal asphyxia [13]. Another study analyzed ROC curve 2 hours
after cardiopulmonary bypass and found that the optimal sensitivity and
specificity for plasma NGAL to predict AKI occurred at a value of 95 ng/mL
in the neonatal group, and for urine NGAL the value was 185 ng/mL [6].
Mishra, et al. [7] that serum NGAL levels at a cutoff value of
139 ng /mL within the first 24 hours of admission to the PICU is highly
sensitive for predicting AKI in critically ill children with septic
shock with a sensitivity of 86% and a relatively poor specificity of
39%.
We conclude that serum NGAL level is elevated within
6 hrs from birth in term neonates with perinatal asphyxia; in
correlation with the evolving HIE severity. High serum NGAL level was
significantly associated with the subsequent diagnosis of AKI in these
neonates. It could thus be speculated that early measurement of this
biomarker in asphyxiated neonates can reliably predict the development
of post-asphyxial acute kidney injury.
Contributors: ENM: conceived and designed the
study and revised the manuscript for important intellectual content;
KSM, ESA: collected the data and drafted the paper; KSM also analyzed
the data and wrote the manuscript; MNH: performed the laboratory work.
The final manuscript was approved by all authors.
Funding: None; Competing interests: None
stated.
References
1. Andreoli SP. Acute renal failure in the newborn.
Semin Perinatol. 2004;28:112-23.
2. Durkan AM, Alexander RT. Acute kidney injury post
neonatal asphyxia. J Pediatr. 2011;158:e29-33.
3. Gupta BD, Sharma P, Bagla JY, Parakh M, Soni J.
Renal failure in asphyxiated neonates. Indian Pediatr. 2005;42:928-34.
4. Karlowicz MG, Adelman RD. Nonoliguric and oliguric
acute renal failure in asphyxiated term neonates. Pediatr Nephrol.
1995;9:718-22.
5. Askenazi DJ, Ambalavanan NS, Goldstein SL. Acute
kidney injury in critically ill newborns: What do we know? What do we
need to learn? Pediatric Nephrol 2009; 24:265-74.
6. Krawczeski CD, Woo JG, Wang Y, Michael R, Ma Q,
Deverajan P. Neutrophil gelatinase-associated Lipocalin concentrations
predict development of acute kidney injury in neonates and children
after cardiopulmonary bypass. J Pediatr. 2011;158;1009-15.
7. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q,
Kelly C, et al. NGAL as a biomarker for acute renal injury after
cardiac surgery. Lancet. 2005; 365: 1231-8.
8. Use and Abuse of the Apgar score. Committee on
fetus and Newborn, American Academy of Pediatrics and Committee on
Obstetrics Practice, American College of Obstetrics and Gynecologists.
Pediatrics. 1996;98:141-2.
9. Ballard JL, Khoury JC, Wedig K, Wang L,
Eilers-walsman BL, Lipp R. New Ballard score: expanded to include
extremely premature infants. J Pediatr. 1991; 119:417-23.
10. Gharehbaghi MM, Peirovifar A. Evaluating causes
of acute renal failure in newborn infants. Pak J Med Sci.
2007;23:877-80.
11. Sarnat HB, Sarnat MS. Neonatal encephalopathy
following fetal distress. A clinical and electroencephalographic study.
Arch Neurol. 1976;33:696-705.
12. Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F,
Lentini P, et al. NGAL: A biomarker of acute kidney injury and
other systemic conditions. Int Urol Nephrol. 2010;42:141-50.
13. Sarafidis K, Tsepkentzi E, Agakidou E, Diamanti
E, Taparkou A, Soubasi V, et al. Serum and urine acute kidney
injury biomarkers in asphyxiated neonates. Pediatr Nephrol. 2012 ;
27:1575-82.
14. Drukker A, Guignard JP. Renal aspects of the term
and preterm infant: a selective update. Curr Opin Pediatr.
2002;14:175-82.
15. Leake RD, Trygstad CW. Glomerular filtration rate
during the period of adaptation to extrauterine life. Pediatr Res.
1977;11:959-62.
16. Bachorzewska-Gajewska H, Malyszko J, Sitniewska
E, Malyszko JS, Pawlak K, Mysliwiec M, et al. Neutrophil-Gelatinase-Associated
Lipocalin (NGAL) correlations with Cystatin C, Serum creatinine and e
GFR in patients with normal serum creatinine undergoing coronary
angiography. Nephrology Dialysis transplantation. 2007; 22:295-6.
17. Koura HM, Galal A, Kandil DM, Elshamaa MF,
Elghorori EA, Khalifa ES. Urinary Neutrophil Gelatinase-Associated
Lipocalin as a marker of disease activity in patients with lupus
nephritis. Int J Acad Res. 2011;3.
|
|
|
 |
|

