|
|
|
Indian Pediatr 2011;48: 235-237 |
 |
Necrotizing Fasciitis Following BCG
Vaccination |
|
Rajoo Thapa, Debkrishna Mallick and Biswajit Biswas
From Department of Pediatrics, The Institute of Child
Health 2, 11, Dr Biresh Guha Street, Kolkata 700 017, India.
Correspondence to: Rajoo Thapa, Department of Pediatrics,
Upstate Medical University Hospitals, 750, E Adams Street, Syracuse, New
York, 13210, USA.
Email: [email protected]
Received: August 6, 2009;
Initial review: September 1, 2009;
Accepted: October 21, 2009.
|
We report a newborn with methicillin-resistant Staphylococcus aureus
mediated necrotizing fasciitis after Bacilli-Calmette-Guerin
vaccination. Radical debridement of the affected area coupled with twice
daily surgical honey dressing and intravenous vancomycin and clindamycin
resulted in satisfactory healing.
Key words: Bacille-Calmette-Guerin vaccine, Necrotizing
fasciitis, Neonate, Staphylococcus aureus.
|
|
Necrotizing fasciitis is
characterized by
vascular thrombosis and necrosis
following rapidly spreading bacterial
infection of the skin, subcutaneous fat and fascia. Systemic dissemination
and toxicity may at times be marked [1]. The most common organisms
implicated include Streptococci of groups B, A and D, Staphylococci,
Gram-negative Enterobacteriae and anaerobes. We described a neonate that
developed Staphylococcus aureus (S. aureus) necrotizing
fasciitis involving the left upper arm following BCG vaccination.
Case Report
A 7 day old previously healthy female neonate, born
spontaneously to a non-consanguineous primipara was initially seen for
fever associated with swelling and redness over the left upper arm. The
baby had received BCG vaccine at our institute, about 18 hours prior to
presentation. The inoculation using 26 G hypodermic needle was strictly
intradermal, as evidenced by a satisfactory 4 mm bleb formation
immediately after the procedure. Sterile saline with cotton was employed
to swab clean the proposed site of vaccination. The mother’s antenatal
period and delivery were uneventful. Examination revealed an excessively
irritable febrile neonate (core temperature 103ºF), with a warm and tender
erythematous swelling, involving the outer aspect of the middle third of
the left arm (approximately 3 cm below the acromion process 2 cm above the
elbow joint). The BCG vaccination site was inflamed. Laboratory
investigations revealed a total leukocyte count of 3,800/mm 3
(N30L62E4M4),
hemoglobin: 19.5 g/dL, platelets: 71,000 /mm3,
C-reactive protein: 109.4 mg/L and micro erythrocyte sedimentation rate:
24 mm (first hour). Considering the clinicolaboratory profile, intravenous
cefuroxime and amikacin were started empirically. The next twelve hours
was characterized by increased toxicity and rapid extension of the
swelling to involve nearly the entire arm with deepening overlying
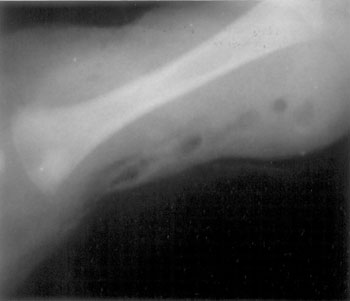
erythema and areas of cutaneous sloughing and necrosis. The radiograph of
the site showed extensive soft-tissue swelling with interposed air-bubbles
(Fig 1). The differentials considered primarily included
neonatal gas-gangrene and necrotizing fasciitis. Radical debridement of
the lesion was done and empirical intravenous clindamycin was added
pending blood cultures. Wound swab culture was sterile for anerobic
organisms; however, positive cultures for methicillin resistant S.
aureus were obtained. Intravenous vancomycin was started in place of
earlier antibiotics; this was continued for 14 days along with intravenous
clindamycin and twice daily surgical honey dressing. Blood cultures
returned sterile after 3 days. Anti-tetanus prophylaxis was instituted
promptly. Laboratory tests directed towards the immune functions of the
baby revealed normal immunoglobulin levels and CD counts. The parents
tested negative for HIV I and II by ELISA. HIV studies were not done on
the neonate. The wound healed satisfactorily by secondary intention
without a skin graft over the next three weeks. The baby was revaccinated
with BCG on the right arm at one month of age and observed for 4
subsequent days. Spirit and cotton swab was used for the preparation of
the proposed vaccination site on this occasion. Finally, she was
discharged home on day 35 of life, feeding satisfactorily with steady
weight gain. She was healthy on follow up.
 |
|
Fig 1 Plain radiograph of the left arm
demonstrating air within the soft tissues around the humerus. |
Discussion
Necrotizing fasciitis is rare in newborns. Commonly
recognized predisposing events include surgery, trauma, ruptured varicella
blisters, and intra-muscular injection sites. The common predisposing
factors in newborns include omphalitis, circumcision, bullous impetigo,
rectal temperature measurement and electrode placement for vital sign
monitoring [2-4]. In the present newborn, no obvious risk factor other
than BCG vaccination in the same arm could be identified. BCG may be
complicated by local edema and axillary adenitis, but necrotizing
fasciitis is rarely reported [5]. This was possibly the result of
bacterial infection and inflammation either by trauma induced by the
procedure of vaccination or due to hypersensitivity to the vaccine itself.
Hypersensitivity to the BCG vaccine could not be excluded in the present
child. Isolation of the pathogenic organism from the lesion confirmed the
etiology.
The IAPCOI, 2007-2008 recommended exclusive use of
sterile saline without local antiseptics (such as spirit) for swabbing the
proposed site of BCG vaccination in neonates [6]. The primary intention of
the recommendation was to avoid instances of contact of the vaccine which
contains live attenuated viable bacilli with antiseptics like spirit which
would otherwise cause rapid inactivation of the same [7]. Certain other
widely cited sources [8] state that if alcohol be used, it must be allowed
to evaporate before the vaccine is given. Sterile saline causes removal of
normal skin flora, including S. aureus by virtue of mechanical
cleansing. It is known that spirit application on the skin kills the
normal skin flora and vegetative organisms like S. aureus by
protein denaturation; however, it does not render the skin surface
absolutely sterile. Alcohol is a highly volatile substance and majority of
it evaporates within few seconds of application on the skin surface. Some
would argue that the application of spirit would lead to the absorption of
the same and would therefore have deleterious effects on the vaccine
containing the live-attenuated tubercle bacilli. Being of a volatile
nature, majority of the spirit would vaporize quickly and whatever little
that enters the deeper skin structures would prove more efficacious
against pathogenic microorganisms that may have entered inadvertently,
without undue inactivation of the vaccine bacilli.
Contributors: RT: critical literature review
and manuscript preparation; DM: patient care and follow up; BB: patient
care and manuscript drafting.
Funding: None.
Competing interests: None stated.
References
1. Legbo JN, Shehu BB. Necrotizing fasciitis: a
comparative analysis of 56 cases. J Natl Med Assoc. 2005;97:1692-7.
2. Hsieh WS, Yang PH, Chao HC, Lai JY. Neonatal
necrotizing fasciitis: a report of three cases and review of the
literature. Pediatrics. 1999;103:e53.
3. Weber DM, Freeman NV, Elhag KM. Periumbilical
necrotizing fasciitis in the newborn. Eur J Pediatr Surg. 2001;11:86-1.
4. Nazir Z. Necrotizing fasciitis in neonates. Pediatr
Surg Int. 2005;21:641-4.
5. Okeniyi JA, Adegbehingbe OO, Dedeke IMF,
Olorunnisola OA, Ogunlesi TA, Oginni LM. Post-BCG Axillary necrotizing
fasciitis. Internet J Pediatr Neonatol. 2006;6.
6. Singhal T, Amdekar YK, Agarwal RK. IAP Guide book on
Immunization, IAP Committee on Immunization 2007-2008. New Delhi: Jaypee
Brothers Medical Publishers; 2009.
7. Tiwari M, John TJ. Skin preparation for BCG
inoculation. Indian Pediatr. 1997;34:1135-6.
8. Park K. Park’s Textbook of Preventive and Social Medicine. Jabalpur:
Bhanot Publishers; 2009.
|
|
|
 |
|

