|
|
|
Indian Pediatr 2013;50:
567-572 |
 |
Hematological Alterations and Thymic Function
in Newborns of HIV-Infected Mothers Receiving Antiretroviral
Drugs
|
|
Rotjanee Wongnoi, Nawaporn Penvieng, Panthong Singboottra, *Doungnapa
Kingkeow,
†Peninnah Oberdorfer, $Pannee
Sirivatanapa and Sakorn Pornprasert
From the Department of Medical Technology, Faculty of
Associated Medical Sciences, *Research Institute for Health Sciences;
†Department of Pediatrics, and $Obstetrics and Gynecology; Faculty of
Medicine; Chiang-Mai University, Chiang-Mai, Thailand.
Correspondence to: Dr Sakorn Pornprasert, Department
of Medical Technology, Faculty of Associated Medical Sciences,
Chiang-Mai University, 110 Intawaroros Road, Chiang-Mai, Thailand,
50200.
Email: [email protected]
Received: July 18, 2012;
Initial review: August 29, 2013;
Accepted: November 21, 2012.
PII: S097475591200623
|
Objectives: To investigate the effects of antiretroviral (ARV) drugs
on hematological parameters and thymic function in HIV-uninfected
newborns of HIV-infected mothers.
Study design: Cross sectional study.
Setting: Chiang-Mai University Hospital,
Chiang-Mai, Thailand.
Participants/Patients: 49 HIV-uninfected and 26
HIV-infected pregnancies.
Methods: Cord blood samples of newborns from
HIV-uninfected and HIV-infected mothers were collected. Hematological
parameters were measured using automatic blood cell count. T-cell
receptor excision circles (TRECs) levels in cord blood mononuclear cells
(CBMCs), CD4+ and CD8+ T-cells were quantified using real-time PCR.
Main Outcome Measures: Hemotological parameters
and thymic function.
Results: Newborn of HIV-infected mother tended to
have lower mean levels of hemoglobin than those of HIV-uninfected mother
(137 ± 22 vs 146 ± 17 g/L, P = 0.05). Furthermore, mean of
red blood cell (RBC) counts and hematocrit and median of TRECs in CD4+
T-cells in the newborns of the former were significantly lower than
those of the latter [3.6 ± 0.7 vs 4.8 ± 0.6 x 1012 cells/L, P
<0.001; 0.40 ± 0.07 vs 0.46 ± 0.05 L/L, P <0.001 and 0.53
(IQR: 0.03-5.76) vs 13.20 (IQR: 2.77-27.51) x 10-3 pg/µL, P
= 0.02, respectively].
Conclusion: ARV drugs altered hematological
parameters and thymic function (TRECs CD4+ T-cells) in HIV-uninfected
newborns of HIV-infected mothers.
Key Words: Adverse effects, Antiretroviral drugs, Hematology,
HIV, Newborn, Thymic function .
|
|
Almost half of the estimated 40 million people
living with HIV are women of childbearing age [1]. The risk of these
women to transmit HIV to their infants is 15-25% when no precautions are
taken [2]. The HIV-mother-to-child transmission (MTCT) rate has
dramatically reduced to be less than 2% with antiretroviral (ARV)
prophylaxis during pregnancy and labor as well as to the infant [3, 4].
The previous studies showed that Zidovudine (ZDV) which is a potent
inhibitor of bone marrow function is associated with hematological
abnormalities not only in mothers, but also in newborns, because this
drug can cross the placental barrier and negatively affect fetal
erythropoiesis [5-8]. Moreover, ZDV-based HAART is commonly associated
with a greater negative impact on hematological parameters than ZDV-free
regimens [9].
The adverse hematological effects of ARV drugs
have been reported in HIV-uninfected infants, especially in their early
life [10]. The frequently adverse hematological effects found are anemia,
neutropenia, lymphocytopenia and thrombocytopenia [7, 11-13].
The thymus is a primary source of naďve T-cells and
plays a key role in establishing and maintaining a peripheral T-cell
pool [14]. Thymus reaches its maximum volume by one year of age [15]. A
production of naďve T-cells by the thymus can be quantified by measuring
T-cell receptor excision circles (TRECs), a DNA fragment formed during
T-cell development. These DNA fragments do not replicate during mitosis
and are thus diluted during cell division [16].
Previous studies demonstrated that both
HIV-proteins and some antiretroviral drugs inhibited progenitor cells
and thymic functions, as indicated by the frequency of TRECs [17-19].
However, an evaluation of hematological and immunological toxicity in
newborn exposed to maternal ARV drugs administered during pregnancy has
been limited. The aims of this study were to measure and compare
hematological parameters and TRECs levels in HIV-uninfected newborn of
HIV-infected mother receiving ARV drugs for prevention of HIV-MTCT with
those of normal control newborn.
Methods
This study was conducted at Chiang-Mai University
Hospital, Chiang-Mai, Thailand. The protocol was approved by the Faculty
of Medicine Ethics Committee, Chiang-Mai University, Chiang-Mai,
Thailand. All pregnant women participating in this study had signed a
written informed consent. To obtain the subjects, the exclusion criteria
for the study were set as follow: women with twin or multiple births,
infected with other micro-organisms, and use of psychopharmaceutical
drugs, illicit drugs, alcohol and tobacco during gestation. From March
to December 2011, 26 HIV-infected and 49 HIV-uninfected pregnant women
were enrolled. These HIV-infected women received ARV drugs [ZDV plus
Lamivudine (3TC) and Lopinavir/Ritonavir (LPV/r)] during pregnancy and
labor every 12 hours with adding of ZDV every 3 hours during labor and
delivered vaginally or elective caesarean section. The following data
were collected from all women: age, gestational age at delivery and mode
of delivery. For the HIV-1 infected women, the following additional data
were collected: antiretroviral prophylaxis (type and timing), CD4 +
T-cell counts (cells/µL) during pregnancy and plasma HIV-1 RNA viral
load measured a week before delivery (log10
copies/mL). All women in our study were given iron and
folate supplementation as recommended by the Thai National Guidelines
for Pregnancies [20]. Diagnosis for HIV-1 infection in infants
born to HIV-1 infected mothers was performed at one and four months of
age using DNA PCR (Amplicor
HIV-1 DNA assay version 1.5, Roche Molecular Systems Inc., USA).
Isolation of cord blood mononuclear cells (CBMCs):
Cord blood samples were drawn from clamped umbilical vein within
5-10 minutes after delivery into ethylenediamine tetraacetic acid
anticoagulation (EDTA) tubes (BD Vacutainer, Franklin Lakes, NJ, USA).
The sample tubes were then shipped to the hematology laboratory, Faculty
of Associated Medical Sciences, Chiang-Mai University within 3 hours.
Upon arrival, hematological parameters were measured using an automated
blood counter (Sysmex KX-21; Sysmex Corporation, Kobe, Japan). Cord
blood mononuclear cells (CBMCs) were isolated using Ficoll-Hypaque
gradient (IsoPrep, Robbins Scientific, Sunnyvale, CA, USA). Cells were
then aliquoted and stored in liquid nitrogen until used.
Separation of CD4 +
and CD8+ T-cells:
It was performed from CBMCs of the 15
HIV-uninfected newborns of HIV-infected mothers and only 12
HIV-uninfected newborns of HIV-uninfected mothers. Frozen CBMCs were
thawed and washed twice in cold phosphate-buffered saline solution. CD4+
and CD8+ T-cells were
separated using a magnetic cell separator (EasySep, STEMCELL
Technologies, USA) according to manufacturers’ instructions. The
separated CD4+ and CD8+
T-cells cells were count on hemacytometer under light microscope using
Turk’s solution.
DNA Preparation and Quantification of TRECs
DNA was extracted from 1.5×10 6
cells of CBMCs, separated CD4+and
CD8+ T-cells using the
NucleoSpin kit (Macherey-Nagel, KG., Duren, Germany) according to
manufacturers’ instructions and was stored at -20oC
until used. TRECs analysis was performed by quantitative
real-time PCR as described by Ometto, et al. [21] with
slightly modification. The DNA amplification was carried out in a 25 µL
reaction mixture containing 5 µL DNA sample or sterile distilled water
as a no template control, 1×real-time PCR Master Mix (Thermo Scientific
ABsoluteTM QPCR ROX Mix,
Surrey, UK), 400 nM each primer (forward, 5'-CACATCCCTTTCAACCATGCT-3';
reverse, 5'-GCCAGCTGCAGGGTTTAGG-3' : GenBank sequence accession number
DQ858179.1) and 200 nM of the fluorogenic probe (5'-ACACCTCTGGTTTTT
GTAAAGG TGCCCAC T-3') conjugated with FAM (6-carboxy-fluorescein) at the
5'-end, and TAMRA (6-carboxy-tetramethilrhodamine) at the 3'-end. The
PCR primers and the fluorogenic probe were specifically designed for the
detection of human TRECs. The amplification was performed in a
Rotor-Gene 6000™ (Corbett Research; Mortlake, New South Wales,
Australia). The mixture was preheated at 95°C for 15 min, followed by 50
cycles at 95°C for 15 sec and 60°C for 1 min. A cycle threshold (CT)
is defined as the PCR cycle at which an increase in the fluorescence
above the baseline signal is first detected. The CT
value is inversely related to the copy number of the target sequence.
TRECs concentrations were calculated from a standard curve of a plasmid
clone containing TRECs which run in parallel with the test. All samples
and TRECs plasmid were run in duplicate. TRECs level in CBMCs was
presented as concentration of TRECs per 1.5 x 106
CBMCs while those in CD4+
and CD8+ T-cell was
presented as concentration of TRECs per cell.
Statistical analysis: Statistical analyses were
performed using SPSS software package (Statistical Package for the
Social Sciences 11.0, Chicago, IL, USA). Characteristics and
hematological parameters were compared between two groups of newborns
using independent samples t test and Fisher’s exact test while
levels of TRECs between the two groups were compared using Mann-Whitney
test. The level of significance for all analyses was set at 0.05.
Results
The clinical data of participants are shown in
Table I. Mean of maternal ages and gestational ages at delivery
were similar between HIV-infected and uninfected women. Most of
HIV-infected and uninfected women delivered vaginally. The HIV RNA viral
loads measured at one week before delivery of HIV-infected women were
less than 40 copies/mL and none of all newborns born to HIV-1 infected
mothers had HIV-infection.
TABLE I Characteristics of HIV-infected and Uninfected Mothers and Their Newborns
|
Characteristics
|
HIV-infected mother
|
HIV-uninfected mother
|
P-value |
|
(n = 26) |
(n = 49) |
|
|
Age at delivery (y) [mean±SD (range)] |
30 ± 7 (17-42) |
27 ± 6 (15-42) |
0.08 |
|
Gestational age at delivery (wks) |
38 ± 2 (33-40) |
38 ± 1 (34-41) |
0.58 |
|
Gestational age at ARV prophylaxis initiation (wks) |
21 ± 5 (14-27) |
Not Relevant |
|
|
CD4+ T-cell count during pregnancy (cells/mL) |
517 ± 188 (186-859) |
Not Relevant |
|
|
HIV RNA load measured at one week before delivery (copies/mL) |
<40 |
Not Relevant |
|
|
Mode of delivery; Vaginal vs Caesarean |
17 : 9 |
39 : 10 |
0.24 |
|
Gender of newborn, Male: Female |
18 : 8 |
24 : 25 |
0.08 |
|
Birth weight of newborn (g) |
2873 ± 461 (2050-3910) |
3029 ± 412 (2250-3950) |
0.18 |
Mean levels of white blood cell (WBC) counts,
absolute neutrophil counts, absolute lymphocyte counts and platelet
counts in newborns of HIV-infected and uninfected mothers did not differ
significantly (Table II). Means of red blood cell (RBC)
counts and hematocrit in newborns of HIV-infected mothers were
significantly lower. On the other hand, newborns of HIV-infected mothers
showed higher mean levels of red cell indices than those of
HIV-uninfected mothers (Table II).
TABLE II Hematological Parameters of Newborns of HIV-infected and Uninfected Mothers
|
Hematological parameters |
Newborn of HIV- |
Newborn of HIV- |
P Value |
|
infected mother (n = 26) |
uninfected mother (n = 49) |
|
|
WBC (x 109 cells/L) |
13.0 ± 5.0 (3.5-24.3) |
14.6 ± 5.6 (5.3-35.1) |
0.24 |
|
Absolute neutrophils (x 109 cells/L) |
7.4 ± 2.6 (2.4-12.3) |
6.5 ± 2.7 (0.8-11.5) |
0.27 |
|
Absolute lymphocytes (x 109 cells/L) |
4.8 ± 2.9 (2.1-12.8) |
5.8 ± 3.1 (2.8-21.2) |
0.19 |
|
RBC (x 1012 cells/L) |
3.6 ± 0.7 (1.7-4.9) |
4.8 ± 0.6 (3.7-6.2) |
<0.001 |
|
Hemoglobin (g/L) |
137 ± 22 (71-166) |
146 ± 17 (104-180) |
0.05 |
|
Hematocrit (L/L) |
0.40 ± 0.07 (0.21-0.51) |
0.46 ± 0.05 (0.36-0.54) |
<0.001 |
|
Mean corpuscular volume (fL) |
113 ± 10 (95-130) |
95 ± 9 (75-110) |
<0.001 |
|
Mean corpuscular hemoglobin (pg) |
38.4 ± 4.3 (30.7-49.4) |
30.48 ± 4.1 (21.2-36.4) |
<0.001 |
|
Mean corpuscular hemoglobin concentration (g/L) |
339 ± 16 (305-380) |
319 ± 20 (271-356) |
<0.001 |
|
Platelet counts (x 109/L) |
318 ± 92 (157-511) |
287 ± 64 (181-422) |
0.12 |
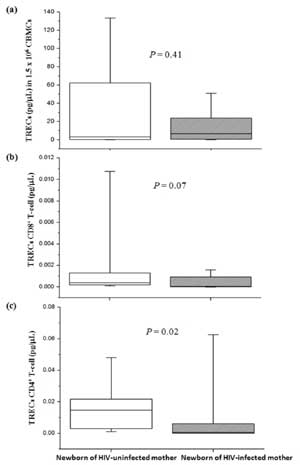
No significant difference in median of TRECs levels
in CBMCs (Fig. 1a) and in CD8 +
T-cell (Fig. 1b) was found between newborns of
HIV-infected mothers and uninfected mothers. However, TRECs levels in
CD4+ T-cell (Fig.
1c) in newborns of HIV-infected mothers were significantly lower
than those of HIV-uninfected mothers.
 |
|
Fig. 1 T-cell receptor excision
circles (TRECs) levels of newborns of HIV-infected and
uninfected mothers. (a) TRECs in CBMCs, (b) TRECs CD8+
T-cell, (c) TRECs CD4+ T-cell. TRECs levels in CBMCs were
analyzed from 26 and 49 newborns of HIV-infected and uninfected
mothers, respectively while TRECs CD8+ and CD4+
T-cell were analyzed from 15 and 12 newborns of HIV-infected and
uninfected mothers, respectively.
|
Discussion
The current study showed that ARV drugs (ZDV plus 3TC
and LPV/r) administered to HIV-infected mother for prevention of
HIV-MTCT altered the hematological parameters of newborns. Furthermore,
the thymic function of these newborns was also impaired as indicated in
the decrease of TRECs CD4+
T-cell. The previous study showed that maternal derived HIV-proteins
diffusing across the placental barrier during pregnancy could reduce
thymic function [19, 22]. In addition, both HIV-proteins and ARV drugs
are known to inhibit progenitor cell function [17, 18]. However, in
present study, the effects of HIV-proteins on thymic function might be
less than those of ARV drugs since maternal viral loads in all
HIV-infected mothers measured at one week before delivery were less than
40 copies/mL.
Our data are reassuring, ARV prophylaxis dose seem to
significantly alter hematological indices because the mean MCV in
newborns of HIV-infected mother was significantly higher than those of
HIV-uninfected mothers. Moreover, some newborns (31%) of HIV-infected
mother had MCV higher than the normal upper limit value (120 fL). In
present study, all HIV-infected mother received ZDV and 3TC, which have
been reported to induce macrocytic anemia [10, 23]. Antiretroviral drugs
are routinely prescribed during the second trimester, in which
hematopoiesis and lymphopoiesis are active, i.e., hepatic hematopoiesis
and lymphopoiesis, spleen development, thymic education and bone marrow
development. The administration of ARV drugs during the critical window
of hematopoiesis and lymphopoiesis may affect the generation of these
precursors [12]. Therefore, an impairement of hematopoiesis and
lymphopoiesis may have contributed to the hematopoietic alteration and
the reduction of thymic output, respectively. The decrease of CD4 +-TRECs
levels observed in the present study was consistent with the previous
study by Clerici, et al. [22] that showed CD4+/45RA/62+
(naďve lymphocytes) in HIV-uninfected newborns of HIV-infected mothers
received ZDV for prevention of HIV-MTCT were significantly lower than
those of newborns of HIV-uninfected mothers. In contrast, Kolte, et
al. [24] showed that thymic size but not thymic function (TRECs CD4+
T-cell) in HIV-uninfected newborns of HIV-infected mothers received ARV
drugs [ZDV/ plus 3TC and LPV/r or Nevirapine (NVP)] for prevention of
HIV-MTCT was significantly lower than those of HIV-uninfected mothers
[24]. While our cohorts were newborns, Kolte’s cohorts were children
with age of 15 months, that was probably when the side effect of ARV
drugs resolved. Moreover, the maternal ethnicities between the two
groups of children were different [24]. There are many parameters that
have been shown to be associated with the hematological variables such
as maternal ethnicity, drug use, maternal CD4+
T-cell count at delivery, mode of delivery and also infant gestation
age, birthweight and sex [25, 26]. In the current study, these factors
were controlled by matching of maternal ethnicity, maternal age at
delivery, gestational age, mode of delivery, fetal sex and birthweight
between the test group and control group.
WBC counts, absolute neutrophil counts, absolute
lymphocyte counts and platelet counts in newborns of HIV-infected
mothers were similar to those of HIV-uninfected mothers (Table
II). These results were consistent with the previous study by
Bunders, et al. [27] that showed the levels of WBC counts,
absolute neutrophil counts, absolute lymphocyte counts and platelet
counts measured at birth in HIV-1/ARV-exposed infants were not different
from those in matched comparison group. However, a lower WBC counts,
absolute neutrophil counts in HIV-1/ARV-exposed infants were observed at
5 weeks of age while a lower level of hemoglobin in these infants were
observed at birth and 5 weeks of age. Thus, further studies are needed
to evaluate how long the hematological alteration and impaired thymic
function persist.
The present study has a limitation in the limited
volume of cord blood collected, thus levels of TRECs in CD4 +
and CD8+ T-cells could be
analyzed in only 12 and 15 samples of newborns of HIV-uninfected and
infected mothers, respectively. Moreover, it was impossible to analyze
the levels of TRECs in memory or naďve CD4+
and CD8+ T-cell
sub-populations (CD45RO+ and
CD45RA+), which are the
immune resources. Although, the hemoglobin and hematocrit in newborns of
HIV-infected mothers were significantly lower than those of
HIV-uninfected mothers. We also found that, mean levels of these two
hematological parameters in both groups were lower than normal range
levels. These lower levels might have caused from the hematologic
genetic disorders such as thalassemia and G-6-PD deficiency, frequently
found in Thai population [28]. However, the hematologic genetic
disorders were not used as a variable factor in our study.
In summary, our study indicates that ARV drugs (ZDV
plus 3TC and LPV/r) for prevention of HIV-MTCH altered the hematological
parameters and impaired thymic function (TRECs CD4 +
T-cell) in newborns of HIV-infected mothers. These phenomena may impact
the quality of life including growth, development, vaccination responses
and susceptibility to infections of infants. Therefore the long-term
effects of these drugs in larger population are needed to be clarified.
Acknowledgments: Staff of Maharaj Nakorn
Chiang-Mai Hospital, Chiang-Mai, Thailand.
Contributors: RW and NP: patient enrolment, data
acquisition, data analysis, laboratory analysis and drafting of
manuscript; PS and DK: data analysis and interpretation and critical
revision of the manuscript; PO, PSV and SP: concept and design, data
acquisition, data analysis and interpretation and critical revision of
the manuscript. All the authors were involved in preparation of the
manuscript.
Funding: The Thailand Research Fund, The
Commission on Higher Education and The National Research University
Project under Thailand’s Office of the Higher Education Commission.
Competing interests: None stated.
References
1. Quinn TC, Overbaugh J. HIV/AIDS in women: an
expanding epidemic. Science. 2005;308:1582-3.
2. Burns DN, Mofenson LM. Paediatric HIV-1 infection.
Lancet. 1999;354:SII1-6.
3. European Collaborative Study. Mother-to-child
transmission of HIV infection in the era of highly active antiretroviral
therapy. Clin Infect Dis. 2005;40:458-65.
4. Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt
J, Diaz C, et al. Combination antiretroviral strategies for the
treatment of pregnant HIV-1-infected women and prevention of perinatal
HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484-94.
5. Baroncelli S, Pinnetti C, Genovese O, Tamburrini
E, Floridia M. Hematological effects of zidovudine prophylaxis in
newborn infants with and without prenatal exposure to zidovudine. J Med
Virol. 2011;83:551-6.
6. El Beitune P, Duarte G. Antiretroviral agents
during pregnancy: consequences on hematologic parameters in HIV-exposed,
uninfected newborn infant. Eur J Obstet Gynecol Reprod Biol.
2006;128:59-63.
7. Feiterna-Sperling C, Weizsaecker K, Buhrer C,
Casteleyn S, Loui A, Schmitz T, et al. Hematologic effects of
maternal antiretroviral therapy and transmission prophylaxis in
HIV-1-exposed uninfected newborn infants. J Acquir Immune Defic Syndr.
2007;45:43-51.
8. Gribaldo L, Malerba I, Collotta A, Casati S,
Pessina A. Inhibition of CFU-E/BFU-E by 3'-azido-3'-deoxythymidine,
chlorpropamide, and protoporphirin IX zinc (II): a comparison between
direct exposure of progenitor cells and long-term exposure of bone
marrow cultures. Toxicol Sci. 2000;58:96-101.
9. Pinnetti C, Baroncelli S, Molinari A, Nardini G,
Genovese O, Ricerca BM, et al. Common occurrence of anaemia at
the end of pregnancy following exposure to zidovudine-free regimens. J
Infect. 2011;63:144-50.
10. Moyle G, Sawyer W, Law M, Amin J, Hill A. Changes
in hematologic parameters and efficacy of thymidine analogue-based,
highly active antiretroviral therapy: a meta-analysis of six
prospective, randomized, comparative studies. Clin Ther.
2004;26:92-7.
11. Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C,
Blanche S. Perinatal antiretroviral treatment and hematopoiesis in
HIV-uninfected infants. AIDS. 2003;17:2053-61.
12. Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz
C, Foca M, et al. Effect of perinatal antiretroviral drug
exposure on hematologic values in HIV-uninfected children: An analysis
of the women and infants transmission study. J Infect Dis.
2006;194:1089-97.
13. Watson WJ, Stevens TP, Weinberg GA. Profound
anemia in a newborn infant of a mother receiving antiretroviral therapy.
Pediatr Infect Dis J. 1998;17:435-6.
14. Bains I, Thiebaut R, Yates AJ, Callard R.
Quantifying thymic export: combining models of naive T cell
proliferation and TCR excision circle dynamics gives an explicit measure
of thymic output. J Immunol. 2009;183:4329-36.
15. Steinmann GG, Klaus B, Muller-Hermelink HK. The
involution of the ageing human thymic epithelium is independent of
puberty. A morphometric study. Scand J Immunol. 1985;22:563-75.
16. Livak F, Schatz DG. T-cell receptor alpha locus
V(D)J recombination by-products are abundant in thymocytes and mature T
cells. Mol Cell Biol. 1996;16:609-18.
17. Clark DR, Repping S, Pakker NG, Prins JM,
Notermans DW, Wit FW, et al. T-cell progenitor function during
progressive human immunodeficiency virus-1 infection and after
antiretroviral therapy. Blood. 2000;96:242-9.
18. Dam Nielsen S, Kjaer Ersboll A, Mathiesen L,
Nielsen JO, Hansen JE. Highly active antiretroviral therapy normalizes
the function of progenitor cells in human immunodeficiency
virus-infected patients. J Infect Dis. 1998;178:1299-305.
19. Nielsen SD, Jeppesen DL, Kolte L, Clark DR,
Sorensen TU, Dreves AM, et al. Impaired progenitor cell function
in HIV-negative infants of HIV-positive mothers results in decreased
thymic output and low CD4 counts. Blood. 2001;98:398-404.
20. Winichagoon P. Prevention and control of anemia:
Thailand experiences. J Nutr. 2002;132:862-6.
21. Ometto L, De Forni D, Patiri F, Trouplin V,
Mammano F, Giacomet V, et al. Immune reconstitution in
HIV-1-infected children on antiretroviral therapy: role of thymic output
and viral fitness. AIDS. 2002;16:839-49.
22. Clerici M, Saresella M, Colombo F, Fossati S,
Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities
in uninfected newborns and children with vertical exposure to HIV.
Blood. 2000;96:3866-71.
23. Eyer-Silva WA, Arabe J, Pinto JF, Morais-De-Sa
CA. Macrocytosis in patients on stavudine. Scand J Infect Dis
2001;33:239-40.
24. Kolte L, Ryder LP, Albrecht-Beste E, Jensen FK,
Nielsen SD. HIV-infected patients with a large thymus maintain higher
CD4 counts in a 5-year follow-up study of patients treated with highly
active antiretroviral therapy. Scand J Immunol. 2009;70:608-13.
25. Bunders M, Thorne C, Newell ML. Maternal and
infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected
children of HIV-1-infected mothers. AIDS. 2005;19:1071-9.
26. Rodriguez EM, Mofenson LM, Chang BH, Rich KC,
Fowler MG, Smeriglio V, et al. Association of maternal drug use
during pregnancy with maternal HIV culture positivity and perinatal HIV
transmission. AIDS. 1996;10:273-82.
27. Bunders MJ, Bekker V, Scherpbier HJ, Boer K,
Godfried M, Kuijpers TW. Haematological parameters of HIV-1-uninfected
infants born to HIV-1-infected mothers. Acta Paediatr.
2005;94:1571-7.
28. Tanphaichitr VS, Mahasandana C, Suvatte V,
Yodthong S, Pung-amritt P, Seeloem J. Prevalence of hemoglobin E, alpha-thalassemia
and glucose-6-phosphate dehydrogenase deficiency in 1,000 cord bloods
studied in Bangkok. Southeast Asian J Trop Med Public Health.
1995;26:271-4.
|
|
|
 |
|

