|
|
|
Indian Pediatr 2011;48:
445-451 |
 |
Whole Body Cooling in Newborn Infants with
Perinatal Asphyxial Encephalopathy in a Low Resource Setting:
A Feasibility Trial |
|
Niranjan Thomas, Koshy C George, Santhanam Sridhar, Manish Kumar,
Kurien Anil Kuruvilla and Atanu Kumar Jana
From the Neonatology Unit, Christian Medical College
Hospital, Vellore, India.
Correspondence to: Dr Niranjan Thomas, Neonatology
Unit, Christian Medical College Hospital, Vellore 632 004, India. Email:
[email protected]
Received: December 8, 2009;
Initial review: February 2, 2010;
Accepted: May 25, 2010.
Published online.
2010 November 30.
PII:S097475590900874-1
|
Objective: To determine the feasibility and safety of whole body
cooling in newborn infants with perinatal asphyxial encephalopathy in a
low resource setting.
Design: Feasibility trial.
Setting: Tertiary care perinatal centre.
Subjects: Infants born at
³ 35 weeks gestation with perinatal
asphyxia were included in the study.
Interventions: Infants were cooled to a rectal
temperature of 33±0.5°C for 72 hours using cloth-covered ice-gel packs.
Vital parameters were monitored continuously.
Outcome measures: The primary outcome was the
achievement of target temperature within 1 hour of initiation of treatment
and maintaining the target temperature for 72 hours. Adverse events and
possible complications of hypothermia were the secondary outcomes
measured.
Results: Twenty infants were included in the study.
The mean time taken to achieve target rectal temperature was 52±25
minutes. The mean rectal temperature during cooling was 32.9±0.11ºC. The
target temperature could be maintained for 72 hours without difficulty in
all babies. Adverse events observed during cooling were thrombocytopenia
(25%), sinus bradycardia (25%), deranged bleeding parameters (20%),
aposteatonecrosis (15%), hyperglycemia (15%), hypoglycemia (10%),
hypoxemia (5%), life-threatening coagulopathy (5%) and death (5%).
Shivering was noted in many of the babies, especially in the initial phase
of cooling.
Conclusion: Whole body cooling in term infants with
perinatal asphyxia is achievable, safe and inexpensive in a low-resource
setting.
Key words: Asphyxia, Feasibility, India, Management, Newborn,
Therapeutic hypothermia.
|
|
Brain cooling initiated within 6 hours
and maintained for 72 hours after an asphyxial insult has been shown to reduce mortality and morbidity among newborn survivors of perinatal asphyxia
[1-7]. Meta-analyses, including the Cochrane systematic review on cooling
for hypoxic ischemic encephalopathy conclude that there is evidence that
therapeutic hypothermia is beneficial to term newborns with hypoxic
ischemic encephalopathy [8-10]. Cooling reduced mortality without
increasing major disability among survivors. However, most studies have
been done in developed countries using expensive equipment.
The present trial was conducted to evaluate whether
whole body cooling (WBC) could be achieved in a low-resource setting using
simple and easily available cooling materials.
Methods
The study was conducted in the neonatal unit of the
Christian Medical College, Vellore between October 2007 to September 2008
after approval from the Institutional Review Board. Term and near-term
babies (gestational age ≥35
weeks) were recruited, and included both inborn and outborn babies with
perinatal asphyxia.
Inborn babies were included in the study if the
following criteria were satisfied: umbilical cord or a postnatal (in the
first hour of life) arterial blood gas pH of < 7.0 or base deficit ≥12 along with any two
of the following: (a) Apgar score ≤5 at 5 minutes; (b)
Ventilation initiated at birth and continued for at least 10 minutes; and,
(c) Perinatal predisposition to perinatal asphyxia (any one) -
intrapartum fetal distress, cord prolapse, placental abruption, maternal
respiratory arrest, and uterine rupture/dehiscence.
The criteria for outborn babies was a history of not
having cried/breathed immediately after birth with evidence of
encephalopathy at admission and any or all of the following features: (a)
not breathing normally at five minutes of birth; (b) given
assistance for breathing soon after birth; (c) flaccid since birth;
(d) poor feeding; and, (e) Apgar score of 5 or less
at 5 minutes.
Babies were excluded from the study if they were small
for gestational age, had chromosomal or major congenital anomaly, refusal
of consent, or inability to start cooling by 5 hours of age. As per the
unit protocol, severely asphyxiated babies (no spontaneous respiration by
30 minutes of life) were not ventilated.
After obtaining informed consent from the parents of
eligible babies, a neurological examination was performed using a
standardized neurological examination that was based on the modified Sarnat criteria and used in the NICHD study [6,11]. The infant warmer was
turned off and cooling achieved by placing 3-5 cloth-covered cooling ice
packs over the back, head, abdomen and the axillae of the baby. These
packs were plastic containers filled with cooling gel used in vaccine
carriers and were stored in the freezer of the refrigerator. These packs
were reused after adequate cleaning.
A rectal probe (Philips-ref no 21090 A or Drager-ref no
4329848-08) to monitor core temperature was inserted 5 cm within the
rectum and connected to a multi-parameter monitor (Philips Intellivue MP20
or Drager vista XL). The desired rectal temperature was 33±0.5°C. The
temperature was continuously monitored and recorded every 15 minutes for 4
hours and then subsequently every hour for 80 hours. The skin temperature
was measured simultaneously. During the cooling process, if the infant’s
rectal temperature approached 33.5°C, more cloth-covered cooling-gel packs
were placed on the body, and these were removed one by one when the rectal
temperature dropped to 33°C. After 72 hours of hypothermia, re-warming was
achieved by removing the packs, turning on the warmer and raising the
temperature of the baby by not more than 0.5°C per hour. The environmental
temperature of the nursery was also recorded during the study period. The
nurse to patient ratio was 1:3. All infants had a central venous line and
arterial access. Continuous monitoring of vital parameters was done. All
treatment including medications (antibiotics, anticonvulsants, inotropes
and sedatives), ventilation, and use of blood products were as per
existing treatment protocols. Neurological examination was repeated at 24
and 72 hours. Serum electrolytes, blood urea, serum creatinine,
prothrombin time (PT), activated partial thromboplastin time (aPTT), liver
enzymes and blood counts were monitored at 0, 24, 48 and 72 hours. Blood
gas was done at 0, 2, 8, 12, 24, 48 and 72 hours. An ECG was obtained if
the heart rate was less than 80/min.
An external data and safety monitoring committee was
notified within 48 hours if any of the following adverse events occurred:
cardiac arrhythmia requiring medical treatment; persistent hypoxemia (transcutaneous
oxygen saturation of 85% or a paO2
<50 mm Hg in spite of a FiO2
of 1 on mechanical ventilation); hypotension despite adequate inotropic
support (dopamine at 10 µg/kg/min and dobutamine at 20 µg/kg/min); skin
changes (sclerema, aposteatonecrosis); thrombocytopenia (<100×103/µL);
life-threatening coagulopathy; arterial thrombosis; hepatic and renal
failure; electrolyte disturbances; and, death.
Statistical analysis: The sample size was
calculated using the design of Gehan (1961). With a 10% precision and a
20% desired effectiveness, the sample size was calculated as 20. The
analysis of the data was done using the SPSS 16.0 software. Mean, median,
mode, standard deviation and frequency were calculated. Tests for
significance used were Pearson’s coefficient and Mann-Whitney U test.
Results
Twenty infants were recruited in the study, of whom 11
(55%) were outborn, and 12 (60%) were female. Maternal and neonatal
details are provided in Tables I and II. Most
mothers were primigravid (90%), had no complications of pregnancy, and
went into spontaneous labour. Fetal heart deceleration was seen in the
majority of inborn babies and 45% were born normally. Cooling was started
at a mean of 3.4 ± 1.2 hours after birth. Majority of the infants
recruited developed moderate encephalopathy.
TABLE I
Maternal Characteristics of the Study Neonates
| Primigravia |
18(90%) |
| Complications of pregnancy |
| Gestational diabetes mellitus |
1 (5%) |
| Pregnancy induced hypertension |
2 (10%) |
| None |
17 (85%) |
| Peripartum complications (inborn)
|
| Fetal heart rate deceleration |
7 (77.7%) |
| Hemorrhage |
1 (11.1%) |
| Meconium stained amniotic fluid |
1 (11.1%) |
| Spontaneous onset of labor |
18(90%) |
| Duration of labour (h) |
13±10 |
| Mode of delivery |
| Normal |
9 (45%) |
| LSCS |
8 (40%) |
| Forceps |
2 (10%) |
| Vacuum |
1 (5%) |
| Inborn No (%) |
9 (45%) |
TABLE II
Characteristics of the Neonates in the Study
|
Mean gestational age (wks) |
38.5 ± 1.3 |
|
Age at starting cooling (h) |
3.4 ± 1.2 |
|
Inborn |
3 ± 1 |
|
Outborn |
3.5 ± 1 |
|
Gender (Inborn: Outborn) |
|
Male (1:1) |
8 (40%) |
|
Female (1:1.4) |
12 (60%) |
|
Birthweight (g) |
3034 ± 518 |
|
Cord blood pH (inborn only) |
6.945 ± 0.118 |
|
Cord blood BE (inborn only)* |
–19.1 ± 3.2 |
|
Moderate encephalopathy, No. (%) |
16 (80%) |
|
Severe encephalopathy, No. (%) |
4 (20%) |
|
*BE: base excess. |
At the start of cooling, the mean rectal temperature
was 36.0±0.8°C and the mean skin temperature was 35.8±0.97°C. The mean
time taken to reach target rectal temperature was 52±25 minutes. A
Kaplan-Meier survival analysis was done with "time to attain target
temperature" as the event. By the end of 15 minutes, 30% had achieved the
target temperature; the median time to event was 45 minutes (95% CI
34.3-55.8). At 60 and 90 minutes from commencement of cooling, only 35%
and 5% of the newborns, respectively had not achieved the target
temperature.
Pearson’s correlation looking at the linear
relationship of birth weight and time taken to achieve target temperature
showed a positive moderate correlation (r=0.545; P<0.05)
suggesting that the larger babies took a longer time to cool. There was no
statistical significance between time taken to cool and gestational age,
gender, type of labor, place and mode of delivery.
The environmental temperature of the nursery during the
study period ranged from 28 to 32oC.
Skin and rectal temperature during cooling: The
mean average rectal temperature and mean average skin temperature during
the period of cooling were 32.9±0.11ºC
and 33.1±0.14 ºC, respectively
(Fig 1a,b). The mean average difference between
rectal and skin temperatures was 0.15±0.13°C. (Fig 1b).
The rate of rewarming after 72 hours of cooling was 0.48 ± 0.07ºC
(Fig 1c). The variation in the mean rectal
temperature from target temperature during the period of cooling was 0.08
± 0.04°C.
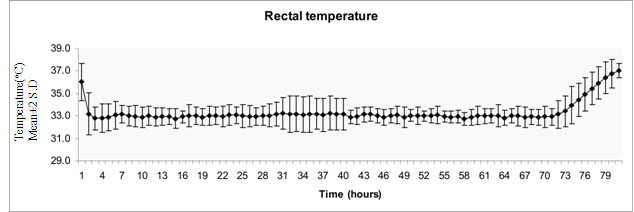 |
|
(a) Rectal temperature |
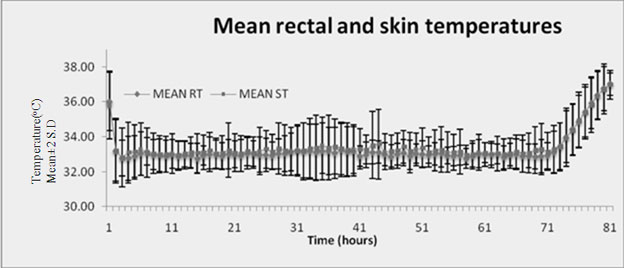 |
|
(b) Trend of rectal and skin temperature |
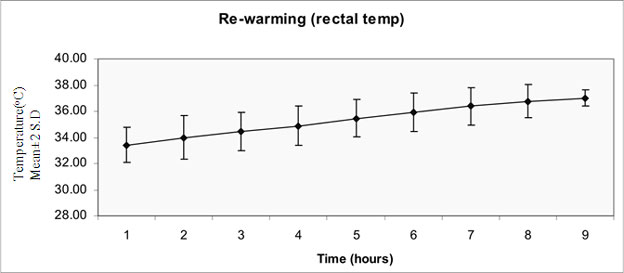 |
|
(c) Trend during re-warming |
|
Fig. 1 Trend of rectal and skin temperature during whole body
cooling.
|
Clinical and laboratory parameters: The mean heart
rate, blood pressure and oxygen saturation during cooling is seen in
Fig. 3. The mean heart rate at the start of cooling was 138±15
per minute while the average mean heart rate during the period of cooling
was 111±5/min. The mean blood pressure at the start of cooling was
54.7±7.9 mm Hg and the average mean blood pressure during the period of
cooling was 55.6 ±1.7 mm Hg. It was noted that many of the babies
shivered, especially in the initial few hours.
There was significant acidosis among inborn babies at
admission. The acidosis corrected in most instances within 12 hours of the
initiation of the procedure, though two babies (10%) had persistent
metabolic acidosis requiring sodium bicarbonate infusion. Serum
electrolytes and blood urea remained within normal limits. Serum
creatinine and blood lactate showed a downward trend as cooling
progressed. Two (10%) infants had hypoglycemia, and 3 (15%) had
hyperglycemia requiring insulin. Liver enzyme levels were elevated
initially but showed a downward trend after 48 hours. The hemoglobin and
packed cell volume dropped marginally and the platelet count showed a
decreasing trend during cooling.
TABLE III
Serious Adverse Events During Cooling
| Adverse events |
No. (%) |
| Cardiac arrhythmias |
Nil |
| Hypoglycemia (blood sugar <45 mg/dL) |
2 (10%) |
| Hyperglycemia requiring insulin |
3 (15%) |
| Thrombocytopenia(<100 ×103/µL ) |
5 (25%) |
| Bleeding |
1 (5%) |
| Aposteatonecrosis |
3 (15%) |
| Hypoxemia |
1 (5%) |
| Hepatic dysfunction |
1 (5%) |
| Oliguria (urine output <0.5 mL/kg/h) |
1 (5%) |
Serious adverse events: The serious adverse events
that occurred during whole body cooling are shown in Table
III. There were no cardiac arrhythmias recorded. However, 5
(25%) babies developed transient sinus bradycardia. Bleeding requiring
transfusions was seen in 1 (5%) infant. This infant had prolonged bleeding
parameters before starting cooling and a subgaleal bleed that worsened
with cooling. Cooling was stopped after 27 hours but the baby continued to
deteriorate and died. Skin changes (aposteatonecrosis) seen in 3 babies
resolved spontaneously. Neurosonogram was done on all infants in the first
week. Periventricular white matter changes were seen in 1(5%) baby while
the rest were normal.
Discussion
This study was designed to look at the feasibility of
whole body hypothermia in a developing country context. We used reusable
ice gel packs obtained from the immunization clinic at no added expense.
These were wrapped in clean cloth to prevent cold injury to the skin. The
only additional cost involved was the cost of the rectal probes (Rs
900/probe) which were reusable. Using this method we could achieve and
maintain the target temperature with ease. Several other researchers have
used cold water/ice gel packs in previous studies either for head cooling
or for inducing whole body hypothermia(12-14).
Compared to this, the standard equipment used in
cooling is expensive: the cooling mattress costs Rs 500,000 while the cool
cap costs Rs 3,500,000. Two studies from Africa also looked at using low
resource methods of cooling; Robertson, et al. [15] used water
bottles filled with cool tap water, and Horn, et al. [16] devised a
servo control fan to maintain hypothermia.
Both studies reported that cooling is possible in a low
resource setting and can be made inexpensive by innovative means.
We have also demonstrated that it is possible to obtain
informed consent and initiate treatment within 5 hours of birth in both
inborn and outborn infants. The mean time to achieve target temperature in
our study was 52±25 minutes. This was similar to the time taken to achieve
cooling in previous studies from China and South Africa [4,15]. Although
many studies do not indicate the time taken to reach the target
temperature, available data suggests that an upper limit of 90 minutes is
more realistic [6]. Though only 65% achieved desired temperature in our
trial within one hour, using this criterion of 90 minutes, 95% of our
newborn infants reached the target rectal temperature.
Wide variations in temperature during cooling not only
increase the adverse effects of cold exposure but also compromise the
degree of neuroprotection, because even small changes (as little as 1-2ºC)
in brain temperature may modulate the extent of hypoxic ischemic damage
[17]. In this trial, the mean rectal temperature was very close to desired
temperature with the mean variation in rectal temperature being only 0.08
± 0.04ºC from the target temperature. It is important to avoid rapid
re-warming as this may offset the neuroprotective effect of hypothermia.
Our re-warming rate was 0.48 ± 0.07 ºC
per hour, demonstrating that slow and smooth re-warming can be achieved
using radiant warmers.
An interesting outcome noted was that the mean average
difference between the rectal and skin temperature during the period of
cooling was only 0.15±0.13ºC; this implies that the skin temperature is
comparable to rectal temperature. A similar finding was reported by Horn,
et al. [14]. However,
further studies are needed to see if using skin temperature alone would
suffice in monitoring babies’ temperature during therapeutic hypothermia.
The results of this feasibility trial are in
concordance with other studies that show that hypothermia is not
associated with serious adverse events like cardiac arrhythmias, prolonged
acidosis, life threatening bleeding or thrombosis. The fall in the heart
rate when the rectal temperature was lowered to the target temperature was
consistent with other reports. Thrombocytopenia seen in five of the
infants could be attributed to cooling. However, there was no significant
clinical bleeding in 4 of these babies. One baby, who had deranged
coagulation profile before the start of cooling, developed disseminated
intravascular coagulation and subsequently died. Hypoglycemia was seen in
two infants requiring higher intravenous carbohydrate intake.
Hyperglycemia requiring insulin was seen in three babies. This may be
attributed to the decrease in insulin secretion during hypothermia [18].
This effect has not been reported in earlier trials involving newborn
infants.
We specifically monitored for skin changes as cloth
covered ice-gel packs were used in the initiation and maintenance of
cooling. At the start of cooling, there was hyperemia in the areas where
the ice-gel packs were placed and this required frequent rotation of the
packs to different areas of the body. During the maintenance phase, no
skin changes were noted. The aposteatonecrosis that was observed in three
babies occurred during the start of cooling and subsequently resolved
without intervention or residual scarring. These have been well described
as an adverse effect of whole body cooling [6,15,19]. Though shivering was
not one of the adverse events monitored, we noted that many of the babies
shivered especially in the initial few hours. The shivering noted in our
study may be related to either under sedation or the method of cooling.
Shivering was also noted by Horn, et al. [16], who also reported
hypomagnesemia in all the babies who shivered. There is evidence from
studies in adult intensive care units to suggest that shivering may be
associated with a worse outcome [20]. Other than sedation, clonidine has
been used for this shivering, both in neonates who have been cooled and in
post operative adult patients [16,21]. A majority of our cooled babies
received phenobarbitone, though we did not routinely give analgesia other
than if ventilated. Sedation during cooling is recommended and there is
animal evidence to show that the neuroprotective effect of hypothermia may
be lost if sedation is not used [22].
This feasibility trial in a developing country using
low cost techniques with careful monitoring of temperature establishes
that this neuro-protective strategy can be achieved safely and at an
affordable cost. There are several issues that need to be addressed in
terms of further research in a low resource setting [23]. These include
the difference in the population in terms of the high incidence of IUGR,
unbooked pregnancies, sepsis and meconium aspiration syndrome, the lack of
transport facilities, which may preclude cooling in outborn babies and the
difference in severity of encephalopathy seen in cooled babies. There is
an urgent need to conduct trials looking at the efficacy and safety of
cooling in this setting specifically addressing these issues.
Contributors: All the authors were involved in the
concept, design, data collection, analysis and drafting of the manuscript.
Funding: CMC, Vellore.
Competing interests: None stated.
|
What is Already Known?
• In developed countries, whole body cooling has
been shown to decrease the risk of death or disability in infants
with moderate to severe hypoxic-ischemic encephalopathy.
What This Study Adds?
• Whole body cooling is feasible and safe in a
low resource setting and can be achieved with minimal additional
cost.
|
References
1. Battin MR, Dezoete JA, Gunn TR,
Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with
head cooling and mild hypothermia after perinatal asphyxia. Pediatrics.
2001;107:480-4.
2. Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of
term infants with head cooling and mild systemic hypothermia (35ºC and
34.5ºC) after perinatal asphyxia. Pediatrics. 2003;111:244-51.
3. Gluckman P, Wyatt J, Azzopardi D, Ballard R, Edwards
D, Ferriero D, et al. Selective head cooling with mild systemic
hypothermia after neonatal encephalopathy: multicentre randomised trial.
Lancet. 2005;365:663-70.
4. Lin Z, Yu H, Lin J, Chen S, Liang Z, Zhang Z. Mild
hypothermia via selective head cooling as neuroprotective therapy in term
neonates with perinatal asphyxia: an experience from a single neonatal
intensive care unit. J Perinatol. 2006;26:180-4.
5. Inder T, Hunt R, Morley C, Coleman L, Stewart M,
Doyle L, et al. Randomized trial of systemic hypothermia
selectively protects the cortex on MRI in term hypoxic-ischemic
encephalopathy. J Pediatr. 2004; 145:835-7.
6. Shankaran S, Laptook A, Ehrenkranz R, Tyson J,
McDonald S, Donovan E, et al. Whole-body hypothermia for neonates
with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574-84.
7. Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday
HL, Juszczak E, et al. TOBY Study Group. Moderate hypothermia to
treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349-58.
8. Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P.
Cooling for newborns with hypoxic-ischaemic encephalopathy. Cochrane
Database Syst Rev. 2007;4: CD003311.
9. Shah P, Ohlsson A, Perlman M. Hypothermia to treat
neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr
Adolesc Med. 2007;161:951-8.
10. Edwards AD, Brocklehurst P, Gunn AJ, Halliday H,
Juszczak E, Levene M, et al. Neurological outcomes at 18 months of
age after moderate hypothermia for perinatal hypoxic ischaemic
encephalopathy: synthesis and meta-analysis of trial data. BMJ.
2010;340:c363.
11. Sarnat HB, Sarnat MS. Neonatal encephalopathy
following fetal distress. A clinical and electroencephalographic study.
Arch Neurol. 1976;33: 696-705.
12. Thorensen M, Whitelaw A. Cardiovascular changes
during mild therapeutic hypothermia and rewarming in infants with
hypoxic-ischemic encephalopathy. Pediatrics. 2000;106:133-4.
13. Horn AR, Woods DL, Thompson C, Elis I, Kroon M.
Selective cerebral hypothermia for post-hypoxic neuroprotection in
neonates using a solid ice cap. S Afr Med J. 2006;96:976-81.
14. Horn AR, Harrison MC, Linley LL. Evaluating a
simple method for neuroprotective hypothermia for newborn infants. J Trop
Pediatr. 2009 Sep 30 [Epub ahead of print] doi:10.1093/tropej/fmp089.
15. Robertson NJ, Nakakeeto M, Hagmann C, Cowan FM,
Acolet D, Iwata O, et al. Therapeutic hypothermia for perinatal
asphyxia in low-resource settings: a pilot randomised controlled trial.
Lancet. 2008;372:801-3.
16. Horn A, Thompson C, Woods D, Nel A, Bekker A, Rhoda
N, et al. Induced hypothermia for infants with hypoxic-ischemic
encephalopathy using a servo-controlled fan: an exploratory pilot study.
Pediatrics. 2009;123:e1090-8.
17. Shankaran S, Laptook A, Wright LL, Ehrenkranz RA,
Donovan EF, Fanaroff AA, et al. Whole body hypothermia for neonatal
encephalopathy: animal observations as a basis for a randomized,
controlled pilot study in term infants. Pediatrics. 2002;110:377-85.
18. Varon J, Acosta P. Therapeutic hypothermia: past,
present, and future. Chest. 2008;133:1267-74.
19. Navarini-Meury S, Schneider J, Bührer C. Sclerema
neonatorum after therapeutic whole-body hypothermia. Arch Dis Child Fetal
Neonatal Ed. 2007;92:F307.
20. Polderman KH Mechanisms of action, physiological
effects, and complications of hypothermia. Crit Care Med.
2009;37:S186-202.
21. Biolatta F, Ferri F, Giovannini F, Pinto G, Rosa G.
Neopam or clonidine in the pharmacological prevention of shivering in
patients undergoing conscious sedation for interventional neuroradiology.
Anaesthesia. 2005; 60:124-8.
22. Thorensen M, Satas S, Loberg EM, Whitelaw A, Acolet
D, Lindgren C, et al. Twenty-four hours of mild hypothermia in
unsedated newborn pigs starting after a severe global hypoxic-ischemic
insult is not neuroprotective. Pediatr Res. 2001;50:405-11.
23. Thayyil S, Costello A, Shankaran S, Robertson NJ.
Therapeutic hypothermia for neonatal encephalopathy: implications for
neonatal units in India. Indian Pediatr. 2009;46:283-9.
|
|
|
 |
|

