|
|
|
Indian Pediatr 2012;49:
543-547 |
 |
Nebulized Hypertonic-Saline vs
Epinephrine for Bronchiolitis: Proof of Concept Study of
Cumulative Sum (CUSUM) Analysis
|
|
Neeraj Gupta, *Ashish Puliyel, Ayush Manchanda and
Jacob Puliyel
From the Department of Pediatrics and Neonatology, St
Stephen’s Hospital, Delhi, India; and
* Tech Guru gonzoBuzz, Singapore.
Correspondence to: Dr Neeraj Gupta, Registrar,
Department of Pediatrics, St Stephen’s Hospital,
Tis Hazari, Delhi 110 054, India.
Email: [email protected]
Received: March 29, 2011;
Initial review: April 26, 2011;
Accepted: August 11, 2011.
Published online: 2011, October 30.
PII: S097475591100270-1
|
Objective: To apply cumulative sum (CUSUM) to monitor a drug trial
of nebulized hypertonic-saline in bronchiolitis. To test if monitoring
with CUSUM control lines is practical and useful as a prompt to stop the
drug trial early, if the study drug performs significantly worse than
the comparator drug.
Design: Prospective, open label, controlled trial
using standard therapy (epinephrine) and study drug (hypertonic-saline)
sequentially in two groups of patients.
Setting: Hospital offering tertiary-level
pediatric care.
Patients: Children, 2 months to 2 years, with
first episode of bronchiolitis, excluding those with cardiac disease,
immunodeficiency and critical illness at presentation.
Interventions: Nebulized epinephrine in first
half of the bronchiolitis season (n = 35) and hypertonic saline
subsequently (n = 29). Continuous monitoring of response to
hypertonic-saline using CUSUM control-charts developed with
epinephrine-response data.
Main outcome measures: Clinical score,
tachycardia and total duration of hospital stay.
Results: In the epinephrine group, the maximum
CUSUM was +2.25 (SD 1.34) and minimum CUSUM was -2.26 (SD 1.34). CUSUM
score with hypertonic-saline group stayed above the zero line throughout
the study.
There was no statistical difference in the
post-treatment clinical score at 24 hours between the treatment groups
{Mean (SD) 3.516 (2.816): 3.552 (2.686); 95% CI: -1.416 to + 1.356},
heart rate {Mean (SD) 136 (44): 137(12); 95% CI: -17.849 to +15.849) or
duration of hospital stay (Mean (SD) 96.029 (111.41): 82.914 (65.940);
95% CI: -33.888 to +60.128}.
Conclusions: The software we developed allows for
drawing of control lines to monitor study drug performance.
Hypertonic-saline performed as well or better than nebulized epinephrine
in bronchiolitis.
Key words: Bronchiolitis, Control limit lines,
CUSUM, Randomized Control Trials, Stopping rule.
Clinical Trial Registration No.: CTRI/2008/091/000233.
|
|
Nebulized epinephrine is commonly used for
treatment of bronchiolitis in children having significant respiratory
distress. Nebulized hypertonic saline is another modality of treatment
[1,2]. It has previously been studied in the context of cystic fibrosis
(CF) [3]. The various mechanisms of action of hypertonic saline have
been reviewed by Mandelberg and Amirav [4]. Nebulized hypertonic saline
with or without bronchodilators has been demonstrated to reduce hospital
stay and improved clinical severity [5,6]. Studies directly comparing
nebulized hypertonic saline with nebulized epinephrine are lacking.
The best answer to this question, of which of the two
alternative interventions is better, can be studied in a double blind
Randomized Control Trial (RCT) comparing the new drug (nebulized
hypertonic saline) against standard therapy (nebulized epinephrine).
However, RCT’s have inherent problems, especially in the context of
trials in children. According to Mc Culloch and colleagues, RCTs involve
difficult blinding, require large samples, long duration, and are very
expensive [7]. Others have noted that it is difficult to recruit cases
[8].
Drug trials often include a provision of interim
analysis and stopping of the trial if large differences between
treatment groups are detected. We did this study to see if instead of
such interim analyses, Cumulative Sum (CUSUM) can be used for continuous
monitoring of the use of the study drug.
Cumulative Sum (CUSUM) is a statistical technique
used in industry for quality control. Positive weights are given for
successes and negative weights for failures such that, as the process
continues, the cumulative score stays close to the zero line. Control
lines are drawn so that if there are more failures or success than would
be expected by chance, the lines would be crossed. These lines can be
drawn using boot-strapping methods, as the sequence of failures and
success depends on chance. By repeatedly reordering the same data
randomly in say 10,000 iterations, the limits of the CUSUM that can
occur by chance can be defined [9]. The highest CUSUM and the lowest
CUSUM is noted for each iteration. The upper limit of CUSUM is
considered the mean upper CUSUM +2 SD and the lower limit is considered
as the mean lower CUSUM-2SD.
CUSUM has been used to study antimicrobial treatment
in neutropenic patients [10] and in the qualitative assessment of
clinical competence [11]. Watkins and colleagues used CUSUM for the
detecting Ross River Virus disease [12]. We have used this novel
statistical tool to test if hypertonic saline was better or worse than
the standard therapy with epinephrine. In this communication we report
the procedure, the software we developed and the results of the study.
Methods
The study was conducted from November 2008 to April
2009. Children aged between two months and two years, presenting with
first episode of acute bronchiolitis and respiratory distress to our
hospital emergency room were enrolled in the study after obtaining
written informed consent from parents. Children with history suggestive
of chronic cardiopulmonary disease, immunodeficiency, past history of
respiratory disease requiring nebulization and critical illness at
presentation were excluded. Children with a history of use of systemic
or nebulized bronchodilators or nebulised hypertonic saline in last 24
hours were also excluded from study. The clinical scoring system
described by Uyan, et al. [13]
was used to clarify severity of respiratory distress
(Web
Table I). As per protocol, children with a clinical
score of 4 or more, and those with oxygen saturation less than 94% in
room air, were advised admission and they were eligible for enrolment in
the study. Enrolled children were treated as usual with standard drug;
nebulized epinephrine (non racemic solution, 1:1000 concentration,1 mL
diluted in 2 mL normal saline) [14] every 6 hours, for first 24 hours.
Children whose clinical score increased by 2 points or more (using
admission score as baseline), or whose heart rate went above 200/minute
were considered treatment failures. If the child improved or did not
deteriorate using the above criteria, the treatment was considered
useful, for the purpose of our study. In case of failure, the drug was
stopped and alternative measures were instituted which could be
escalated up to ventilation. Nebulized epinephrine was given during the
first 3 months of study. CUSUM data were analyzed to look for failures
in standard therapy, to draw control limit lines. In CUSUM, the data
were arranged as either treatment success (S) or failure (F) with
standard drug (epinephrine). The scores of +1 and -1 were given for each
successful or failed treatment respectively. The weightage of each
success and failure was calculated such that the cumulative sum of
original data, comes to zero. Using boot-strapping technique and by
random rearrangement of the sequence we looked at the CUSUM scores in
10,000 iterations. This helped to examine the limits that occur purely
on account of chance changes in the sequence of successes. CUSUM scores
using the boot-strapping method provided the upper and lower control
lines. The mean upper CUSUM score + 2SD provided the upper limit and the
mean lower CUSUM score -2SD provided the lower limit.
Children enrolled in the second half of the
bronchiolitis season (the next 3 months after the initial 35 patients
were recruited to receive standard therapy) received nebulized
hypertonic (3%) saline, 3 mL every 6 hours. Clinical score and heart
rate were monitored. Success and failures were measured by the same
criteria as with epinephrine. The CUSUM score with nebulized hypertonic
saline was plotted. It was decided a priori that if
failure rate crossed the lower CUSUM control line, the trial would be
stopped immediately. If however, successes were more and it crossed the
upper control line, the study would be continued till the end of
bronchiolitis season and it would be clear that new drug is superior to
standard therapy. In the absence of suitable software, we developed
custom-built open-source software [15] which allows inputting of any
initial series data (epinephrine in the study). The software does the
boot-strapping and calculates the limits for the control line and the
stopping rule.
Conventional statistical methods were employed to
examine differences between groups. To compare means and the confidence
intervals, we used statistical software (Confidence Interval Analysis)
(CIA) [16]. This study was approved by the Hospital Ethics Committee.
Results
64 patients were enrolled in the study; 35 received
nebulized epinephrine and subsequently, 29 received nebulized hypertonic
saline. Details of children in the two groups are given in Table
I. There was no statistically significant difference in the age,
sex distribution, clinical score and heart rate at admission. There was
no significant difference in the duration of stay between the groups.
TABLE I Population Characteristics of The Two Groups
|
Patient characteristic |
Epinephrine |
Hypertonic
|
95% CI for difference |
P
|
|
group(n=35)
|
saline group(n=29) |
between means |
value |
|
Male: Female |
25:10 |
23:6 |
|
|
|
Age, mo (SD) |
7.1 (6.58) |
5.27 (3.82) |
-0.886 to + 4.646 |
<0.05 |
|
Clinical score at admission (Mean, SD) |
8.2 (2.57) |
7.55 (2.28) |
-0.549 to + 1.909 |
<0.05 |
|
Heart rate at admission (/min.) (Mean, SD) |
149 (41) |
143 (16) |
-10.168 to + 22.168 |
<0.05 |
|
Clinical score at 24 hours (Mean, SD) |
3.5 (2.81) |
3.5 (2.68) |
-1.416 to + 1.356 |
<0.05 |
|
Heart rate at 24 hours (/min.) (Mean, SD) |
136 (44) |
137 (12) |
-17.849 to + 15.849 |
<0.05 |
|
Duration of stay in hours (Mean, SD) |
96.03 (111.41) |
82.91 (65.94) |
-33.888 to + 60.128 |
<0.05 |
With the initial trial with epinephrine, there were 5
children with ‘treatment failure’ (2 with worsening of clinical score
and 3 with tachycardia). The resultant weightage for success and failure
were +0.286 and –1.714, respectively. The highest CUSUM during the trial
using epinephrine was +5.429 and the lowest CUSUM was –0.571.
Boot-strapping was done with 10,000 iterations. Increasing iterations
beyond this number did not significantly alter the CUSUM maximum and
minimum values. The maximum CUSUM was +2.253 (SD 1.342) and minimum was
–2.259 (SD 1.337). 2 SD (standard deviation) was added to the maximum
CUSUM, and 2 SD was subtracted from the minimum CUSUM values, to draw
the control lines as shown in Fig. 1. The CUSUM score
while using hypertonic saline stayed above the zero.
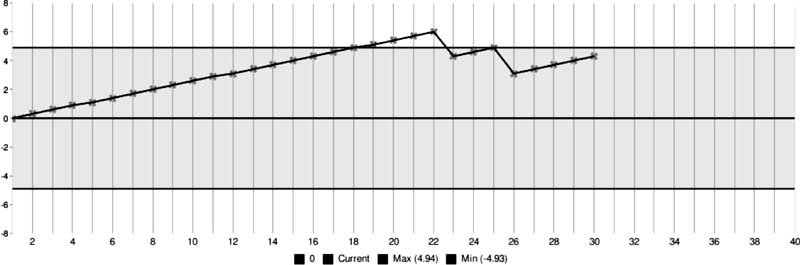 |
|
Fig. 1 Real time CUSUM plot with nebulized hypertonic
saline with CUSUM control lines.
|
Discussion
Many clinical trials include some strategy for
interim analysis and early stopping, if large differences between
treatment groups are detected. This design feature can reduce the study
participants’ exposure to inferior treatment in addition to saving time
and resources. Interim analyses are done at different predetermined
points during the study. Instead of using the traditional form of
interim analysis, we used the CUSUM to continuously monitor the new drug
(nebulized hypertonic saline). In this ‘proof-of-concept study’ we
monitored the new drug with a view to terminate the study if the drug
was less useful than standard therapy with epinephrine in preventing
deterioration in bronchiolitis. Other than using CUSUM for this stopping
rule, we employed conventional statistical methods to compare the two
treatment groups. We found that CUSUM monitoring is possible in the
context of clinical trials.
The software we have developed allows CUSUM to be
deployed easily in a number of clinical contexts besides drug trials.
The use of this software in evaluating the competence of ophthalmic
surgeons for cataract surgery has been published [17].
In this study, we found that nebulized hypertonic
saline was at least as good as nebulized epinephrine in the treatment of
acute bronchiolitis. Deterioration after hospitalization was no more
frequent with hypertonic saline than with epinephrine. Previous studies
using hypertonic saline have also found it more useful than placebo
(normal saline) [1,5,18] or
salbutamol alone [19]. This is the first study directly comparing
nebulized hypertonic saline with nebulized epinephrine.
It must be emphasized that epinephrine is not used as
standard therapy, in the treatment of bronchiolitis universally. In our
proof-of-concept study we used epinephrine as ‘standard therapy’ for the
first 3 months to act as controls, and study drug in the second half of
the bronchiolitis season. We did not determine the sample size for this
study as we felt that the stopping rule would govern numbers of patients
recruited for the trial drug. However, we should have used conventional
methods to calculate sample size to determine the number of children
receiving standard therapy. The study drug trial recruitment could also
be continued till the required sample is reached, unless the study has
to be curtailed early for breeching the stopping rule. Another major
limitation of the study was the comparison of two interventions at
different time periods in a sequence. Changes other than the drug might
have influenced the results during two different time periods.
This novel technique of using CUSUM helps to stop
trials early, if the study drug performs worse than the comparator. This
will help limit the risks to study subjects. The parameters to be
monitored using CUSUM could be side effects or therapeutic benefits. It
can be used for comparisons against placebo or against standard therapy.
The new tool can be used within open label RCT to allow for continuous
monitoring of the trial. The idea for a clinical CUSUM calculator was
taken from industry (quality control mechanism) to provide a simple
method to monitor a drug trial. Paradoxically, industry itself may find
the simple software we developed, handy for quality control.
Compared to RCT this tool, however, does not allow
blinding and randomization easily. Though use of CUSUM provides the
advantage of continuous monitoring of the trial, it cannot replace the
standard double blind RCTs. We believe that the software we developed
for the study allows easy boot-strapping and drawing of control lines
and thus has potential for use in many clinical situations.
Contributors: NG, AP, AM and JP conceived the
project, NG conducted the clinical trial. AP helped NG and JP in the
statistical analysis and development of software.NG, AM and JP were
responsible for the write up. JP will act as guarantor.
Funding: None; Competing interests: None
stated.
Note: The software used in this study was custom
built for the study. It can be downloaded free from the internet.
|
What is Already Known?
• Nebulized hypertonic saline is better than
placebo in bronchiolitis.
What This Study Adds?
• Nebulized hypertonic saline is at least as
good as treatment with nebulized epinephrine in bronchiolitis.
• A software for easy calculations of CUSUM has been
developed that can help monitor new therapies in real time.
|
References
1. Mandelberg A, Tal G, Witzling M, Someck E, Houri
S, Balin A, et al. Nebulized 3% hypertonic saline solution
treatment in hospitalized infants with viral bronchiolitis. Chest.
2003;123:481-7.
2. Tal G, Cesar K, Oron A, Houri S, Ballin A,
Mandelberg A. Hypertonic saline/epinephrine treatment in hospitalized
infants with viral bronchiolitis reduces hospitalization stay: 2 years
experience. Isr Med Assoc J. 2006;8:169-73.
3. Wark PA, McDonald V. Nebulized hypertonic saline
for cystic fibrosis. Cochrane Database Syst Rev. 2000;2:CD001506.
4. Mandelberg A, Amirav I. Hypertonic saline or high
volume normal saline for viral bronchiolitis: mechanisms and rationale.
Pediatr Pulmonol. 2010;451:36-40.
5. Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen
TP. Nebulized hypertonic saline solution for acute bronchiolitis in
infants. Cochrane Database Syst Rev. 2008;4:CD006458.
6. Mathew JL. What works in bronchiolitis? Indian
Pediatr. 2009;46:154-8.
7. McCulloch P, Taylor I, Sasako M, Lovett B, Griffin
D. Randomised trials in surgery: problems and possible solutions. BMJ.
2002;324:1448-51.
8. Yeung V. Clinical trials in children. In:
Pediatric Drug Handling. Available from
http://www.pharmpress.com/shop/samples/paeditaric_sample_chapter.pdf.
Accessed October 12, 2009.
9. Taylor WA. Change-Point Analysis: A Powerful New
Tool for Detecting Changes. Available from: URL
http://www.variation.com/cpa/tech/changepoint.html. Accessed October 11,
2009.
10. Kinsey SE, Giles FJ, Holton J. Cusum plotting of
temperature charts for assessing antimicrobial treatment in neutropenic
patients. BMJ. 1989;299:775-6.
11. Ravn LI, Sprehn M, Pedersen CB. The Cusum score.
A tool for evaluation of clinical competence. Ugeskr Laeger.
2001;163:3644-8.
12. Watkins RE, Eagleson S, Veenendaal B, Wright G,
Plant AJ. Applying cusum-based methods for the detection of outbreaks of
Ross River virus disease in Western Australia. BMC Med Inform Decis Mak.
2008;8:37.
13. Uyan AP, Ozyurek H, Keskin M, Afsar Y, Yilmaz E.
Comparison of two different bronchodilators in the treatment of acute
bronchiolitis. Internet Journal of Pediatrics and Neonatology.
2003;3;1.
14. Beck R, Elias N, Shoval S, Tov N, Talmon G,
Godfrey S, et al. Computerized acoustic assessment of treatment
efficacy of nebulized epinephrine and albuterol in RSV bronchiolitis.
BMC Pediatr. 2007;7:22.
15. Foresee: CUSUM Calculator for Clinical Control
(4-C Software). Available from http://jacob.puliyel.com/calculator/.
Accessed August 7, 2011.
16. Confidence Interval Analysis (CIA) Software.
Available from http://www.som.soton.ac.uk/research/sites/cia/. Accessed
August 7, 2011.
17. Puliyel A, Puliyel J. CUSUM for monitoring
competency: computer software is useful for bootstrapping and real time
CUSUM plotting. Br J Ophthalmol. 2011;95:295-6.
18. Kuzik BA, Al-Qadhi SA, Kent S, Flavin MP, Hopman
W, Hotte S, et al. Nebulized hypertonic saline in the treatment
of viral bronchiolitis in infants. J Pediatr. 2007;151:266-70.
19. Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et
al. Nebulized hypertonic saline/salbutamol solution treatment in
hospitalized children with mild to moderate bronchiolitis. Pediatr Int.
2010;52:199-202.
|
|
|
 |
|

