|
|
|
Indian Pediatr 2012;49:
537-542 |
 |
Incidence of Acute Kidney Injury in
Hospitalized Children
|
|
Poonam Mehta, Aditi Sinha, Abdus Sami, Pankaj Hari,
*Mani Kalaivani, Ashima Gulati, Madhulika Kabra, Sushil K Kabra, Rakesh
Lodha and Arvind Bagga
From the Departments of Pediatrics and *Biostatistics,
All India Institute of Medical Sciences, New Delhi, India.
Correspondence to: Dr Aditi Sinha, Division of
Nephrology, Department of Pediatrics, All India Institute of Medical
Sciences, Ansari Nagar, New Delhi 110029, India.
E-mail: [email protected]
Received: September 3, 2011;
Initial review: October 7, 2011;
Accepted: November 09, 2011.
Published online: Dec 17, 2011.
SII : S097475591100739-1
|
Objective: To determine the incidence and outcome of acute kidney
injury (AKI) in hospitalized patients.
Design: Prospective, observational.
Setting: Tertiary care center in North India.
Participants/patients: Inpatients, 1 month to
18-yr-old.
Intervention: None.
Main Outcome Measures: Incidence of AKI based on
the serum creatinine criteria proposed by the AKI Network.
Results: During February to September 2008,
thirty nine of 108 (36.1%) critically ill patients and 34 of 378 (9.0%)
patients who were not critically ill developed AKI (P <0.001);
the respective incidence densities were 45.1 and 11.7 cases/1000 patient
days, respectively. The maximal stage of AKI was stage 1 in 48 (65.8%)
patients, stage 2 in 13 (17.8%) and stage 3 in 12 (16.4%) patients; 11
(15.1%) required dialysis. Patients with AKI had a significantly longer
duration of hospital stay (9 days vs 7 days, P<0.02) and
higher mortality (37% vs 8.7%; hazard ratio, HR 2.73; 95% CI
1.64, 4.54). Independent risk factors for AKI were young age (HR 0.89;
95% CI 0.83, 0.95), shock (HR 2.65; 95% CI 1.32, 5.31), sepsis (HR 3.64;
95% CI 2.20, 6.01), and need for mechanical ventilation (2.18; 95% CI
1.12, 4.26). Compared to patients without AKI, the mortality was higher
for AKI stage 2 (HR 5.18; 95% CI 2.59, 10.38) and stage 3 (HR 4.34; 95%
CI 2.06, 9.16). Shock was an independent risk factor for mortality (HR
10.7; 95% CI 4.96, 22.98).
Conclusions: AKI is common in critically ill
children, especially younger patients with septicemia and shock, and
results in increased hospital stay and high mortality.
Key words: Acute Kidney Injury Network, Acute tubular
necrosis, Dialysis, India.
|
|
Acute kidney injury (AKI) is an
important condition in hospitalized patients, associated with adverse
short- and long term outcomes [1,2]. Mortality rates in critically ill
children with AKI are high, ranging between 9% and 67% [3,4] and
increase if complicated by multiorgan failure, organ transplantation and
acute respiratory distress syndrome
[1, 5]. Most cases of incident AKI represent acute
tubular necrosis (ATN) that is secondary to hypovolemia, sepsis or the
use of nephrotoxic agents [1,6].
Recent reviews emphasize that disparities in the
definition of AKI have resulted in large variations in reported
incidence and outcomes [1,6]. The definition and staging of AKI has been
recently standardized using the RIFLE classification proposed by the
Acute Dialysis Quality Initiative Group [7], and the one suggested by
the Acute Kidney Injury Network (AKIN) [8]. These classifications have
been examined in hospitalized adults [9,10] and children [3,11-15], and
found useful in characterizing AKI.
Most pediatric studies on the incidence of AKI are
limited to the developed countries [3,11-12,14,16] and are based on
retrospective analysis of records [3,12]. Given that the spectrum of AKI
differs in developing countries and that retrospective ascertainment of
diagnosis is difficult, we aimed to prospectively determine the
incidence and course of AKI in children hospitalized at a tertiary care
center in North India.
Methods
This prospective study was carried out on consecutive
patients, between the ages of 1 month and 18 years, admitted to the
Pediatric inpatient and Pediatric intensive care unit (PICU) at the All
India Institute of Medical Sciences, New Delhi from February to
September 2008. The following patients were excluded: (i)
chronic kidney disease stage 5 (estimated glomerular filtration rate <15
mL/min/1.73 m 2) [17];(ii)
bilirubin level >5 mg/dL; (iii) hospital stay for less
than 24 h; (iv) known AKI at admission, with serum
creatinine >1.5 mg/dL; and (v) serum creatinine not
done at admission or at 48 h.
The study was approved by the Institute Ethics
Committee. Following informed parental consent, information regarding
the diagnosis, comorbidities and duration of hospital stay were
recorded. The Pediatric Index of Mortality score version 2 (PIM2) was
computed for patients admitted to the PICU [18]. Patients were
classified as (critically ill) if they were admitted to the PICU,
required mechanical ventilation or vasopressor support (need for
dopamine and/or dobutamine at a dose exceeding 10 µg/kg/minute, and/or
adrenaline at any dose for management of hypotension). Patients who did
not meet these criteria were considered (not critically ill).
Serum levels of creatinine were estimated on Hitachi
717 autoanalyzer by modified Jaffe method [19], at admission and
thereafter every 24±6 h for 3 consecutive days in all patients.
Subsequently, the estimation was done at daily intervals in patients
with AKI and in the critically ill. In those not critically ill, but
having risk factors (features of dehydration, congestive heart failure
or shock; therapy with diuretics or nephrotoxic agents [20]; new onset
sepsis), these levels were determined every 48±6 h until discharge.
Based on the AKIN criteria, AKI was defined as abrupt
(within 48 h) reduction in kidney function with an increase in
creatinine level [8]. The illness was categorized as stage 1 (increase
of creatinine by ³0.3
mg/dL, or to 1.5-1.99 times baseline), stage 2 (increase to 2–2.99 times
baseline) and stage 3 (increase to ³3
times baseline, or ³4
mg/dL with an acute rise of >0.5 mg/dL) [8]. The urine output criterion
was not used for defining or staging AKI. Shock was defined in presence
of tachycardia, feeble pulses, cool peripheries, hypotension (blood
pressure <-2 SD for age and sex) or capillary filling time >3 seconds
[21]. Sepsis was the presence of systemic inflammatory response syndrome
with suspected or proven infection [21].
The patients were evaluated to ascertain the etiology
of AKI, its progression and need for dialysis. They were followed until
discharge and the outcome was examined in relation to the maximal stage
of AKI. ATN was defined as renal dysfunction, in a setting of diarrhea
with dehydration, blood loss, cardiac dysfunction, sepsis, burns or use
of nephrotoxic agents, in the absence of active urinary sediment. Acute
interstitial nephritis was considered in patients with leukocyturia and
suggestive renal histology.
Complete recovery was defined as normal urinalysis
and blood pressure, and normal serum creatinine for age (0.2-0.4 mg/dL
for infants; 0.3-0.7 mg/dL for 1-12 yr; 0.5-1.0 mg/dL for 13-18 yr)
[22]. Partial recovery was the presence of hypertension, abnormal
urinalysis (>1+ proteinuria, urine protein to creatinine ratio >0.2
mg/mg; >5 leukocytes or red cells per high power field) or elevated
serum creatinine. Patients requiring maintenance dialysis were
classified as dialysis dependent.
Statistical analysis: The incidence of AKI in
children is approximately 5% among non-critically ill [16,23] and 30% in
critically ill [1,5]. In order to estimate these incidence rates at 95%
confidence, and precision of 2.5% for the non-critically ill and 9% for
critically ill, the required sample sizes were 304 and 104,
respectively.
Results were analyzed using STATA software version 11
(College Station, TX). Continuous data were expressed as median
(interquartile range, IQR) and categorical variables as number (%). The
incidence density (95% confidence interval, CI) was the number of cases
per 1000 patient days. Patient characteristics between groups were
compared using Fisher exact test or Wilcoxon rank-sum test. Mortality
was compared in patients with and without AKI using the log rank test.
Risk factors for AKI and mortality were examined using Cox proportional
hazard analysis and reported as hazard ratio (95% confidence interval,
CI).
Results
Of 613 patients screened, 127 were excluded (Fig.
1), including 28 patients admitted with a diagnosis of AKI,
secondary to rapidly progressive glomerulonephritis (9), hemolytic
uremic syndrome (7), dehydration (7) and septicemia (5).
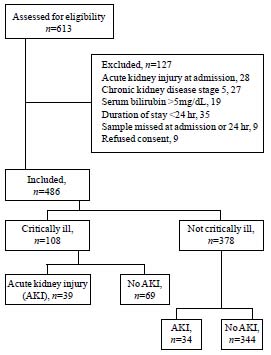 |
|
Fig.1 Flow chart showing the details of patients
recruited into the study.
|
Of 486 patients, 108 (22.2%) were critically ill and
378 (77.8%) were non-critically ill (Table I). Among
critically ill patients, the PIM2 scores at admission were 10.7
(5.4-21.6). The common diagnoses at admission were pneumonia or asthma
(22.6%), malignancy (16.7%), neurological illness (14.6%) and renal
diseases (9.5%).
TABLE I Baseline Clinical and Biochemical Characteristics (N=486)
|
Characteristics |
Critically ill |
Non-critically
|
|
(n=108) |
ill (n=378) |
|
Age, mo
|
43 (9-72) |
48 (12-96) |
|
Girls
|
43 (39.8) |
132 (34.9) |
|
Blood creatinine, mg/dL |
0.5 (0.4-0.6) |
0.5 (0.4-0.6) |
|
Diagnosis at admission |
|
Pneumonia, asthma |
31 (28.7) |
79 (20.9) |
|
Malignancy |
19 (17.6) |
62 (16.4) |
|
Neurological illness |
23 (21.3) |
48 (12.7) |
|
Heart disease |
10 (9.3) |
9 (2.4) |
|
Renal disease*
|
1 (0.9) |
45 (11.9) |
|
Liver disease |
4 (3.7) |
25 (6.6) |
|
Gastroenteritis |
4 (3.7) |
12 (3.2) |
|
Connective tissue disease |
1 (0.9) |
13 (3.4) |
|
Immunodeficiency |
1 (0.9) |
10 (2.6) |
|
Dengue |
4 (3.7) |
7 (1.9) |
|
Malaria |
1 (0.9) |
6 (1.6) |
|
Others |
9 (8.3) |
62 (16.4) |
|
Values for continuous variables are expressed as median (interquartile
range); categorical variables are expressed as number (%); *nephrotic
syndrome, glomerulonephritis, obstructive uropathy
|
Incidence and etiology
Seventy three (15.0%) patients, including 39 who were
critically ill, developed AKI at a median (IQR) of 2 (2-3) days. The
incidence of AKI was 36.1% in the critically ill patients and 9.0% in
the non-critically ill (P <0.001). The overall incidence density
of AKI was 19.4 (95%, CI 15.42, 24.39) cases per 1000 patient days. The
incidence density was higher for critically ill patients (45.1; 95% CI
3.3, 61.78 cases per 1000 patient days) than the non-critically
ill (11.7; 95% CI 8.37, 16.4 cases per 1000 patient days).
The most common etiology of AKI was considered to be
ATN (n=70, 95.9%), both in critically ill (n=39) and
non-critically ill patients (n=31). Other causes were acute
interstitial nephritis (n=2) and bladder outlet obstruction (n=1).
Sepsis (n=42, 60%) and shock (n=38, 54.3%) were the chief
predisposing conditions for ATN; other factors, alone or in combination,
were nephrotoxic agents (n=14), congestive heart failure (n=7),
diarrheal dehydration (n=6) and blood loss (n=3).
Clinical features
Table II shows that patients with AKI
were younger than those without AKI (P=0.002). They also had
significantly higher frequencies of shock, sepsis and need for
vasopressor support and mechanical ventilation. Critically ill patients
with AKI tended to have higher PIM2 scores than those without AKI (P=0.08).
TABLE II Characteristics of Patients With and Without Acute Kidney Injury
|
Characteristic |
Acute kidney
|
No acute
|
| |
injury |
kidney injury
|
|
(n=73) |
(n=413) |
|
Age, mo
|
24 (7-60) |
48 (12.5-96) |
|
Girls |
30 (41.1) |
145 (35.1) |
|
Diagnoses at admission |
|
|
|
Pneumonia |
21 (28.8) |
82 (19.9) |
|
Malignancy |
11 (15.1) |
70 (17.0) |
|
Neurological illness |
10 (13.7) |
61 (14.8) |
|
Heart disease |
4 (5.5) |
15 (3.6) |
|
Renal disease* |
7 (9.6) |
39 (9.4) |
|
Mechanical ventilation# |
35 (48.0) |
58 (14.0) |
|
Shock# |
38 (52.1) |
98 (23.7) |
|
Sepsis# |
42 (57.5) |
124 (30.0) |
|
Vasopressor support# |
29 (39.8) |
62 (15.0) |
|
PIM2 score (in PICU) |
13.1 (7.9-25.2) |
9.1 (4.9-16.7) |
|
Mortality# |
27 (37.0) |
36 (8.7) |
|
Values for continuous variables are expressed as median (interquartile
range); categorical variables are expressed as number (%); *Nephrotic
syndrome, glomerulonephritis, obstructive uropathy; #P<0.001;
$P=0.002. |
AKI stage 1 was detected in 60 patients, stage 2 in
11 and stage 3 in two patients. Five and 7 patients from stage 1 AKI
progressed to stages 2 and 3, respectively, while three patients
advanced from stage 2 to stage 3. The maximal stages of AKI were stage 1
in 48, stage 2 in 13 and stage 3 in 12 patients. Eleven (91.7%) patients
with AKI stage 3 required peritoneal dialysis (n=8) or
hemodialysis (n=3), starting 3 to 20 days after hospital
admission. The median duration of hospital stay was 9 (6-13) days for
patients with AKI compared to 7 (5-10) days for those without AKI (P=0.02).
Outcome
Sixty three patients died after a median duration of
8 (range 2-49) days. A higher proportion of critically ill patients died
(60 of 108; 55.5%) compared to those not critically ill (3 of 378;
0.8%). The mortality in patients with and without AKI was 37.0% and
8.7%, respectively. The mortality in patients with AKI stage 1 (n=7,
14.6%) was lower compared to stage 2 (n=11, 84.6%) and stage 3 (n=9,
75%) (P<0.001).
Among 41 survivors with AKI stage 1, 22 showed
complete recovery. Of 19 patients with partial renal recovery, 11 had
abnormal urinalysis, and 5 each showed hypertension and elevated serum
creatinine. Of 2 patients with AKI stage 2, one each showed complete and
partial renal recovery at discharge. One patient each with AKI stage 3
showed complete and partial renal recovery, and one was dialysis
dependent.
On Cox regression analysis, independent risk factors
for AKI included young age and presence of shock, sepsis and need for
mechanical ventilation (P<0.001) (Table III). On
univariate analysis, risk factors for mortality were shock (HR 11.93;
95% CI 5.64, 25.25; P<0.001) and presence of AKI (2.73; 95% CI
1.64, 4.54; P<0.001). Compared to patients without AKI, the risk
of mortality was higher for AKI stage 2 (HR 5.18; 95% CI 2.59, 10.38;
P<0.001) and stage 3 (HR 4.34; 95% CI 2.06, 9.16; P<0.001),
but not for stage 1 (HR 1.23; 95% CI 0.54, 2.80; P 0.62). On Cox
regression, shock was the only independent risk factor for mortality (HR
10.7; 95% CI 4.96, 22.98; P<0.001).
TABLE III Risk Factors for Acute Kidney Injury
|
Risk factor |
Hazard ratio (95% confidence interval) |
|
Unadjusted |
Adjusted |
|
Age, y
|
0.91 (0.81, 0.97)* |
0.89 (0.83, 0.95)* |
|
Female gender
|
1.23 (0.80, 2.02) |
1.16 (0.72, 1.87) |
|
Shock
|
2.85 (1.80, 4.52)** |
2.65 (1.32, 5.31)* |
|
Sepsis |
2.75 (1.73, 4.37)** |
3.64 (2.20, 6.01)** |
|
Mechanical ventilation
|
4.12 (2.60, 6.52)** |
2.18 (1.12, 4.26)* |
|
*P<0.05, **P <0.001 |
Discussion
This prospective study, from a referral center in
North India, found that the incidence density of AKI in hospitalized
patients was 19.4 cases per 1000 patient days. The incidence was 4-fold
higher in critically ill patients compared to the non-critically ill.
While most patients initially showed stage 1 disease, there was
progressive kidney dysfunction, and 15.1% required renal replacement
therapy. Younger patients and those with sepsis, shock and mechanical
ventilation were at increased risk for AKI. The presence of AKI resulted
in prolonged hospital stay and a four-fold higher mortality, especially
among patients with AKI stages 2 and 3.
Two recently proposed classifications, the RIFLE [7]
and AKIN [8] criteria have been validated as diagnostic and prognostic
tools in critically ill adult patients with AKI [9, 10]. Studies in
critically sick children, using the RIFLE [12] or its pediatric
modification, pRIFLE [3, 14], show that the incidence of AKI varies from
10% to 58%. Based on the former, Schneider, et al. [12] reported
that 339 of 3396 (10%) patients admitted to a PICU in Los Angeles had
AKI. The AKIN criteria have been used in three recent studies in
children [13-15]. Zappitelli, et al reported that the incidence
of AKI in hospitalized children treated with aminoglycosides was 20% by
the AKIN definition and 33% by pRIFLE [14]. Although these criteria were
used in other studies, [14,15] neither study reported on its incidence.
Using similar criteria, we found that more than one-third of all
critically ill patients showed incident AKI. While there is limited
information on AKI among hospitalized, non-critically ill patients
[16,19], the present study showed an incidence of 9%. Based on present
and previous reports, independent risk factors for AKI were young age,
hypotension and sepsis, and the need for mechanical ventilation
[1,3,15].
The etiology of AKI in children varies in developed
and developing countries. In the former, AKI follows major surgeries,
complications associated with malignancies and the use of nephrotoxic
drugs [1,6]. In developing countries, hemolytic uremic syndrome, severe
systemic infections, diarrheal dehydration, and postinfectious
glomerulonephritis constitute important causes [7, 24]. Since the
present study aimed to determine the incidence of AKI in hospitalized
children, we excluded patients with a known diagnosis of AKI at
admission. However, it is notable that apart from the 73 patients (15%)
with incident AKI, 28 patients (5.5%) were admitted with a diagnosis of
AKI secondary to hemolytic uremic syndrome, septicemia, rapidly
progressive glomerulonephritis, and dehydration. Comparable findings
were reported by Schneider, et al. [12] where the rates of AKI at
admission and that developing during hospital stay were 5.7% and 10%,
respectively.
The occurrence of AKI has significant implications,
with considerable short and long term morbidity and mortality [1,2].
Almost 6-45% of critically sick patients with incident AKI require renal
replacement therapy, as was confirmed in the present study [5,11,13,15].
The risk of mortality varies, reflecting the heterogeneous criteria used
for definition, and the spectrum and severity of the underlying illness.
Compared to rates between 9 to 67% in various reports [3,4,11-13,15],
mortality was 37% in the present study. Furthermore, we found that the
risk of mortality was higher in patients with AKI stages 2 and 3 than in
those without kidney injury. While data from multiple studies suggest
that AKI is an independent risk factor for mortality [5,11,13,15], these
findings were not confirmed in the present study. We speculate that the
severity of underlying illness and the presence of hypotension in these
patients predisposed to death more strongly than incident AKI.
Almost half of the patients with stage 1 AKI
recovered completely, and the other survivors showed partial recovery at
the time of discharge. While the present study was underpowered to
examine outcomes in different grades of AKI, it is possible that minor
grades of renal injury have fewer implications than more significant
changes in renal function.
The present study has multiple limitations. Precise
measurements of urine output were not done and the diagnosis of AKI was
based only on levels of serum creatinine. While some studies [10,11,25]
suggest that criteria based on urine output have little effect on
assignment of the final AKI stage and its association with outcomes,
this might have resulted in underreporting of the incidence. Secondly,
this study was performed at a single center, on patients who were sicker
and many had chronic morbidities. It would be necessary to confirm the
incidence of AKI in hospitalized children in other settings. Neonates
were excluded in this study since their susceptibility and etiology of
AKI is considerably different from older infants and children. Exclusion
of patients with serum creatinine above 1.5 mg/dL may have resulted in
erroneous exclusion of patients with unknown but early chronic kidney
disease. The present study was not powered to examine risk factors for
mortality, and larger studies that address these risk factors are
necessary. Finally, the lack of information on outcomes after discharge
does not allow assessment of the impact of mild AKI on short and
long-term renal function.
This prospective study provides data on the incidence
of AKI in hospitalized children. It emphasizes that the incidence of AKI
is high in patients who are critically sick, especially young children
with shock, sepsis and those requiring mechanical ventilation. The
presence of AKI resulted in prolonged hospital stay and increased
mortality. Further studies are required to examine the short- and
medium-term impact of AKI on renal outcome.
Contributors: AB and ASi conceived and
designed the study, analyzed the data and were directly involved in
paper writing; AB would act as guarantor; PM and AS were responsible for
data collection; PM also contributed to analysis and drafting of the
paper; MK provided statistical inputs; RL, MKb, SKK, PH and AG
participated in protocol development and implementation of the study.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
• Acute kidney injury (AKI) is common in
hospitalized critically ill children and is associated with
increased mortality
What This Study Adds?
• Incident AKI affects almost one-third of
critically sick and 10% of non-critically sick hospitalized
children.
• Risk factors for AKI include young age,
shock, sepsis and need for mechanical ventilation
• Higher stages of AKI are associated with increased
mortality and prolonged hospital stay.
|
References
1. Basu RK, Prasad DP, Wong H, Wheeler DS. An update
and review of acute kidney injury in pediatrics. Pediatr Crit Care Med.
2011;12:339-47.
2. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S,
Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients
after acute renal failure. Kidney Int. 2006;69:184-9.
3. Palmieri T, Lavrentieva A, Greenhalgh D. An
assessment of acute kidney injury with modified RIFLE criteria in
pediatric patients with severe burns. Intensive Care Med.
2009;35:2125-9.
4. Kendirli T, Ekim M, Ozcakar ZB, Yüksel S, Acar B,
Oztürk-Hiismi B, et al. Renal replacement therapies in pediatric
intensive care patients: Experiences of one center in Turkey. Pediatr
Int. 2007;49:345-8.
5. Askenazi DJ, Ambalavanan N, Hamilton K, Cutter G,
Laney D, Kaslow R, et al. Acute kidney injury and renal
replacement therapy independently predict mortality in neonatal and
pediatric noncardiac patients on extracorporeal membrane oxygenation.
Pediatr Crit Care Med. 2011;12:e1-6.
6. Cerdá J, Bagga A, Kher V, Chakravarthi RM. The
contrasting characteristics of acute kidney injury in developed and
developing countries. Nat Clin Pract Nephrol. 2008;4:138-53.
7. The ADQI workgroup, Bellomo R, Ronco C, Kellum JA,
Mehta RL, Palevsky P. Acute renal failure: Definition, outcome measures,
animal models, fluid therapy and information technology needs: The
Second International Consensus Conference of the Acute Dialysis Quality
Initiative (ADQI) Group. Crit Care. 2004;8:R204-12.
8. Acute Kidney Injury Network, Mehta RL, Kellum JA,
Shah SV, Molitoris BA, Ronco C, et al. Acute Kidney Injury
Network: Report of an initiative to improve outcomes in acute kidney
injury. Crit Care. 2007;11:R31.
9. Hoste EA, Clermont G, Kersten A, Venkataraman R,
Angus DC, De Bacquer D, et al. RIFLE criteria for acute
kidney injury are associated with hospital mortality in critically ill
patients: A cohort analysis. Crit Care. 2006;10:R73-82.
10. Joannidis M, Metnitz B, Bauer P, Schusterschitz
N, Moreno R, Druml W, et al. Acute kidney injury in critically
ill patients classified by AKIN versus RIFLE using the SAPS 3 database.
Intensive Care Med. 2009;35:1692-1702.
11. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn
KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically
ill children with acute kidney injury. Kidney Int. 2007;71:1028-35.
12. Schneider J, Khemani R, Grushkin C, Bart R. Serum
creatinine as stratified in the RIFLE score for acute kidney injury is
associated with mortality and length of stay for children in the
pediatric intensive care unit. Crit Care Med. 2010;38:933-9.
13. Ozcakar ZB, Yalcinkaya F, Altas B, Ergün H,
Kendirli T, Ates C, et al. Application of the new classification
criteria of the Acute Kidney Injury Network: a pilot study in a
pediatric population. Pediatr Nephrol. 2009;24:1379-84.
14. Zappitelli M, Moffett BS, Hyder A, Goldstein SL.
Acute kidney injury in non-critically ill children treated with
aminoglycoside antibiotics in a tertiary healthcare centre: A
retrospective cohort study. Nephrol Dial Transplant. 2011;26:144-50.
15. The Turkish Society for Pediatric Nephrology
Acute Kidney Injury Study Group, Duzova A, Bakkaloglu A, Kalyoncu M,
Poyrazoglu H, Delibas A, et al. Etiology and outcome of
acute kidney injury in children. Pediatr Nephrol. 2010;25:1453-61.
16. Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn
KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute
kidney injury varies with definition interpretation. Clin J Am Soc
Nephrol. 2008;3:948-54.
17. Schwartz GJ, Munoz A, Schneider MF, Mak RH,
Kaskel F, Warady BA, et al. New equations to estimate GFR
in children with CKD. J Am Soc Nephrol. 2009;20:629-37.
18. Slater A, Shann F, Pearson G. Pediatric Index of
mortality (PIM) Study group. PIM 2: A revised version of the Pediatric
Index of Mortality. Intensive Care Med. 2003; 29:278-85.
19. Bowers LS, Wong ET. Kinetic serum creatinine
assay II. A critical analysis and review. Clin Chem. 1980;26:555-61.
20. Jones DP, Chesney RW. Nephrotoxins. In.
Avner ED, Harmon WE, Niaudet P, Yoshikawa N, eds. Pediatric
Nephrology, Sixth edition. Berlin Heidelberg: Springer-Verlag; 2009.
p.1275-96.
21. Goldstein B, Giroir B, Randolph A. International
Pediatric Sepsis Consensus Conference: Definitions for sepsis and organ
dysfunction in Pediatrics. Pediatr Crit Care Med. 2005;6:2-8.
22. Ceriotti F, Boyd JC, Klein G, Henny J, Queraltó
J, Kairisto V, et al. IFCC Committee on Reference Intervals and
Decision Limits (C-RIDL). Reference intervals for serum creatinine
concentrations: assessment of available data for global application.
Clin Chem. 2008;54:559-66.
23. Hou SH, Bushinsky DA, Wish JB, Cohen JJ,
Harrington JT. Hospital-acquired renal insufficiency: A prospective
study. Am J Med. 1983;74:243-8.
24. Srivastava RN, Bagga A, Moudgil A. Acute renal
failure in north Indian children. Indian J Med Res. 1990;92:404-8.
25. North East Italian Prospective Hospital Renal
Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI) Investigators, Cruz
DN, Bolgan I, Perazella MA, Bonello M, de Cal M, et al. North
East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney
Injury (NEiPHROS-AKI): Targeting the problem with the RIFLE Criteria.
Clin J Am Soc Nephrol. 2007;2:418-25.
|
|
|
 |
|

