|
|
|
Indian Pediatr 2010;47: 599-605 |
 |
Antibiotic Prophylaxis Following
Urinary Tract Infection in Children: A Systematic
Review of Randomized Controlled Trials |
|
Joseph L Mathew
Advanced Pediatrics Centre, PGIMER,
Chandigarh 160 012, India.
Email:
[email protected]
|
|
Relevance
As many as 2-3% boys and 8-11%
girls are reported to have urinary tract
infection(UTI) during childhood(1,2). Despite rapid
diagnosis and treatment, there is a reported 5% risk
of long term damage(3) owing to recurrence and
consequent renal scarring with its (later)
complications. Therefore, it is customary to
prescribe long term antibiotic prophylaxis following
UTI, irrespective of the presence or absence of
‘risk factors’(4) such as anatomic malformations,
vesico-ureteral reflux (VUR), female gender etc.
Most guidelines recommend prophylaxis in the
management of UTI(5,6). However, data supporting
such recommendations is limited and based on
outdated research; therefore it is relevant to
examine current best evidence on the subject.
This systematic review addresses
the question: "In children with urinary tract
infection (population), does antibiotic
prophylaxis (intervention), prevent
recurrence, renal scarring, long-term complications,
etc (outcome), as compared to no prophylaxis
(comparison)?
Current Best Evidence
Literature search was undertaken
for systematic reviews and randomized controlled
trials (RCT) comparing antibiotic prophylaxis versus
no prophylaxis in children following episode(s) of
UTI, irrespective of underlying renal condition(s).
Trials comparing different antibiotics (against each
other) were not considered. Outcomes of interest
were recurrence of UTI, new or worse renal scarring,
long term complications, cost, and antibiotic
resistance.
Medline search (25 May 2010)
using the Mesh terms for ‘UTI’ and ‘antibiotic
prophylaxis’, with Limits "Meta-Analysis,
Randomized Controlled Trial, Review, All Child: 0-18
years", yielded 66 citations. Simultaneous
Cochrane Library search using "urinary
tract infection AND antibiotic" in "Record
Title", yielded 10 Cochrane reviews/protocols, 4
other reviews, 46 clinical trials, 1 HTA and 4
economic evaluations. Two Cochrane reviews appeared
relevant(7,8). One examined antibiotic
prophylaxis(7), but does not include all currently
available trials; it also combined older trials
(with inappropriate UTI definitions) and recent
trials. The other review(8) examined interventions
for children with VUR only. Non-Cochrane
reviews(9,10) were not up-to-date, necessitating a
fresh systematic review.
Fifteen citations were
shortlisted from the preliminary search and
examination of References for additional trials.
Among these, 10 were excluded for the following
reasons: (i) not RCT (n=4)(11-14), (ii)
definition of UTI not consistent with current
definition (n=3)(15-17), (iii)
cross-over study without randomization
component(18), (iv) trial in children with
VUR but not after UTI(19) and (v) description
of ongoing RCT, but data not available(20). Thus,
data from five RCTs (21-25) comprise current best
evidence.
TABLE I
Characteristics of Included Trials
|
No |
Setting |
Study
design |
Participants |
N
(Px/ No Px) |
Follow-up |
Outcomes |
Ref |
| 1 |
Australia |
MC, DB,
PCT |
N=576
< 18y with >1 episode symptomatic UTI* |
288/288
CTX
(10mg/kg/d) vs
placebo |
Every
3mo × 12 mo |
Recurrence of symptomatic UTI, UTI with fever,
hospitalization for UTI, hospitalization for
other causes, antibiotic for concomitant
illness, scarring, UTI with resistant bacteria,
adverse events |
21 |
| 2 |
France |
MC, RCT |
N=225
1mo-3y with VUR (I-III) diagnosed after UTI**with
CTX sensitive organism |
103/122
CTX (10mg/kg/d) vs
nothing x 18 mo
|
Every 3
mo ×
18 mo |
Recurrence of UTI, symptoms, scarring, VUR
status |
22 |
| 3 |
Italy |
MC, RCT |
N=338
2mo-7y with 1st UTI#
+ VUR (I-III) |
211/127
CTX or CA (15mg/kg/d)
vs nothing |
Every
1-2 mo × 12 mo |
Recurrence of UTI, new renal scarring, adverse
events, recurrence with resistant organism. |
23 |
| 4 |
Italy |
MC, RCT |
N=100<30 mo with VUR (II-IV) diagnosed after 1st
episode of APN† |
50/50
CTX (5-10mg/kg/d) or NF (2mg/kg) vs nothing x 2y |
4 y |
Recurrence of APN, new/worse scarring |
24 |
| 5 |
USA,
Canada, Spain, Peru |
MC, RCT |
N=236
3mo-18 y with APN‡
and VUR (I-III) |
100/118
CTX
(5-10mg/kg/d) or NF
(1.5mg/kg/d) vs nothing |
Every
3mo × 1 y |
Recurrence of UTI, renal scarring |
25 |
|
APN=acute pyelonephritis, BS=bag specimen,
CA=co-amoxyclav, CS=catheter specimen, CFU=colony
forming units, CTX=cotrimoxazole equivalent,
DB=double blinded, FU=follow-up, MC=multicentric,
MSS=mid-stream sample, NF=nitrofurantoin,
PCT=placebo controlled trial, Px=prophylaxis,
SPT=supra-pubic tap, UTI=urinary tract
infection, VUR=vesico-ureteral reflux;
*Defined as symptoms consistent with UTI and
positive culture (any growth of single
pathogenic organism on SPT or ≥104CFU/ml from CS
or ≥105CFU/ml from morning MSS); **Defined as
single organism ≥105CFU/ml from MSS or BS (if
non toilet-trained); #Defined as fever >38 deg
C + pyuria + single organism ≥105 CFU/ml (2
concordant consecutive results); †Defined as
fever of unknown origin + bacteriuria and
leucocytes in urine + ≥106 CFU/ml in two
different samples collected by MSS or CS; ‡Defined as fever, pyuria with
≥ 10WBC/hpf, ≥105 CFU/ml in CS or MSS + scan evidence of APN. |
Table I summarizes the
characteristics of included trials. All used co-trimoxazole
in standard doses; three also included
co-amoxyclav(23) or nitrofurantoin(24,25). Only
one(21) was placebo-controlled. Two trials(22,25)
included only children with VUR; one(24) enrolled
participants after an episode of acute
pyelonephritis. Two trials(21,25) included children
up to 18 years of age. Various outcomes were
examined including recurrence of UTI and scarring.
One trial(24) examined renal scarring, but did not
present results. Risk of bias (Table II)
was low for three trials(21,23,24). The trials
reported sample size calculations; one could not
recruit the planned number(21) and another
calculated sample-size for 70% power(23).
TABLE II
Risk of Bias and Other Design Characteristics of Included Trials (Cochrane Risk of Bias Tool)
|
Trial |
Randomization |
Allocation
concealment |
Blinding |
ITT analysis |
Risk of bias |
Sample size |
Ref |
|
1 |
Adequate |
Adequate |
Adequate |
Yes |
Low |
Yes |
21 |
|
2 |
Unclear |
Unclear |
Inadequate |
No |
High |
Yes |
22 |
|
3 |
Adequate |
Adequate |
Inadequate |
Yes |
Low |
Yes (power set at
70%) |
23 |
|
4 |
Adequate |
Adequate |
Partial |
Yes |
Low |
Yes |
24 |
|
5 |
Unclear |
Unclear |
Inadequate |
No |
High |
Yes |
25 |
|
ITT = intention-to-treat. |
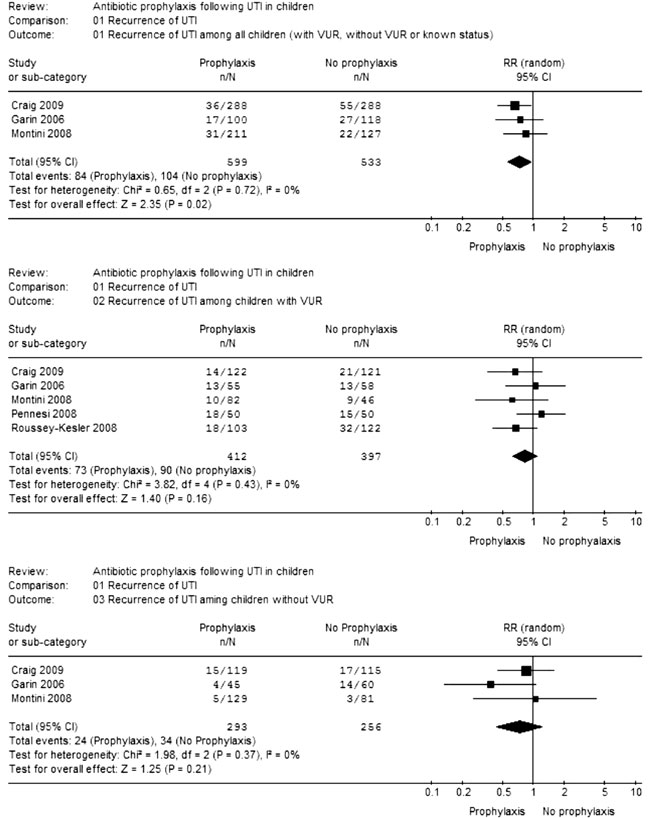
Meta-analysis showed that risk of
UTI recurrence (Fig.1) was reduced
with antibiotic prophylaxis when all children (with
VUR, without VUR and unknown status) were considered
together (RR=0.73; CI=0.56-0.95; 3 trials; 1132
participants; I 2=0%).
However, there was no benefit of prophylaxis when
children with VUR (RR=0.82; CI=0.62-1.08; 5 trials;
809 participants; I2=0%) and without VUR (RR=0.72;
CI=0.43-1.20; 3 trials; 549 participants; I2=0%)
were examined separately. Antibiotic prophylaxis did
not prevent new/worsening renal scarring in children
with VUR (RR=2.64; CI=0.53-13.03; 1 trial; 113
participants), without VUR (RR=0.67; CI=0.13-3.48; 1
trial; 105 participants) and both groups combined
(RR=1.00; CI=0.49-2.03; 3 trials; 667 participants;
I2=0%) (Fig.2). The risk of adverse
events/side effects increased significantly with
antibiotics (RR=3.08; CI=0.02-549.95; 2 trials; 914
participants; I2=92%). Likewise, children on
prophylaxis appeared to have higher risk of UTI
recurrence with a resistant organism (RR=8.60;
CI=0.86-85.81; 3 trials; 190 participants; I2=82%).
|
 |
|
Fig.1 Meta-analysis
of data on recurrence of UTI
|
|
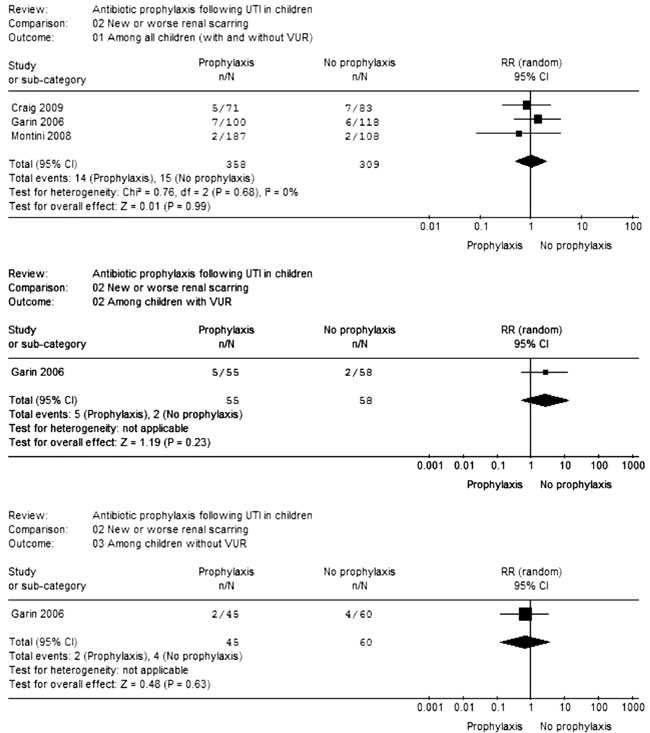 |
|
Fig.2
Meta-analysis of data on new/worse renal
scarring. |
Critical Appraisal
Recommendations for antibiotic
prophylaxis following UTI were based on the
expectation of increased risk of recurrence, and
consequent long-term renal damage (through scarring)
including hypertension etc. Supporting data was
limited in quantity (4 RCT with 117 participants)
and (methodological) quality. Additionally, the
trial definitions of UTI are not currently accepted.
Some recent trials with better (though not ideal)
methodology have reported different results,
necessitating better designed RCT and systematic
review of evidence. Examination of current best
evidence also raises the following issues:
The definition of UTI is a
critical issue in RCT of antibiotic prophylaxis; all
the older trials(15-18) defined UTI in a manner not
accepted currently, including some participants
without ‘true’ UTI. In contrast, all the recent
trials(21-25) have used stringent definitions,
reducing the risk of false-positives. Therefore,
combining the older with the recent trials may be
inappropriate.
Is concomitant fever a necessary
component of UTI (to further reduce the risk of
false-positives)? Although this will increase
specificity, in real life, UTI is often treated even
if fever is absent. It can also be argued that with
modern practices of urine specimen collection and
microbiologic criteria for UTI, fever strengthens
the diagnosis, but may not be necessary. Therefore
this review has not looked at symptomatic/febrile
UTI separately.
Should children with and without
VUR be considered separately? The argument in
favour is that the risk of UTI recurrence is higher
with VUR, hence these children should be viewed
differently. The arguments against are that the risk
does not appear to be very different with and
without VUR, the relationship between VUR and renal
scarring is not clear(24), diagnosis is often made
after UTI, and VUR often resolves over time(26,27).
In clinical practice, prophylaxis is often initiated
empirically irrespective of presence/absence of VUR.
Therefore this review has examined antibiotic
prophylaxis separately among children with and
without VUR, and also both groups combined.
Prolonged antibiotic therapy is
not without risk; this includes individual as well
as community risk in terms of adverse events/side
effects and encouraging antimicrobial
resistance(21,23,25). The latter risk has increased
over the decades; therefore justification for
antimicrobial prophylaxis today, should be stricter
than three decades back (when the original trials
were conducted). Based on this, the balance of
current evidence leans away from antibiotic
prophylaxis.
Current evidence is unable to
identify subgroup(s) of children who may benefit
from antibiotic prophylaxis. This is an important
issue because most trials exclude children with
complex congenital malformations and/or higher
grades of VUR (especially V). It is possible that
the balance between benefit and harm of
antimicrobial prophylaxis in children at greater
risk of complications is different from those
included in clinical trials, necessitating
individualized decisions in the absence of evidence.
Therefore, future research should focus on these
specific high(er) risk groups rather than routine
UTI (with or without lower grades of VUR).
Good evidence of the impact of
compliance (or otherwise) to long term prophylaxis
is not available. Lack of compliance could
apparently reduce the beneficial effect of
prophylaxis. Whereas, better compliance during
clinical trials could suggest greater benefit than
in real life (efficacy versus effectiveness).
Extendibility
None of the trials comprising
current best evidence were conducted in our country;
however, there is no reason to suspect that Indian
children behave differently in terms of UTI or risk
of recurrence and/or complications. Hence, the
evidence can be extended to our setting. On the
other hand, the risk of inappropriate antibiotic
usage and consequent antimicrobial resistance could
be a bigger problem in our setting, necessitating
greater caution.
Funding: None.
Competing interests: None
stated.
|
EURECA Conclusion in the
Indian Context
• Antibiotic prophylaxis
following UTI does not appear to prevent
recurrence of infection and/or renal scarring
in children with or without VUR, considered
separately.
• Antibiotic prophylaxis could result in
increased risk of recurrence with resistant
organisms.
|
References
1. Hellstrom A, Hanson E, Hansson
S, Hjalmas K, Jodal U. Association between urinary
symptoms at 7 years old and previous urinary tract
infection. Arch Dis Child 1991; 66: 232-234.
2. DeMuri GP, Wald ER. Imaging
and antimicrobial prophylaxis following the
diagnosis of urinary tract infection in children.
Pediatr Infect Dis J 2008; 27: 553-554.
3. Coulthard MG, Lambert HJ, Keir
MJ. Occurrence of renal scars in children after
their first referral for urinary tract infection.
BMJ 1997; 315: 918-919.
4. Hellerstein S. Urinary tract
infections: old and new concepts. Pediatr Clin North
Am 1995; 42: 1433-1457.
5. Bagga A, Babu K, Kanitkar M, Srivastava
RN; Indian Pediatric Nephrology Group, Indian
Academy of Pediatrics. Consensus statement on
management of urinary tract infections. Indian
Pediatr 2001; 38: 1106-1115.
6. American Academy of Pediatrics
Committee on Quality Improvement, Subcommittee on
Urinary Tract Infection. Practice parameter: the
diagnosis, treatment, and evaluation of the initial
urinary tract infection in febrile infants and young
children. Pediatrics 1999; 103: 843-852.
7. Williams GJ, Wei L, Lee A,
Craig JC. Long-term antibiotics for preventing
recurrent urinary tract infection in children.
Cochrane Database Syst Rev 2006; 19; CD001534.
8. Hodson EM, Wheeler DM, Smith
GH,Craig JC, Vimalachandra D. Interventions for
primary vesicoureteric reflux. Cochrane Database
Syst Rev 2007; 3: CD001532.
9. Mori R, Fitzgerald A, Williams
C, Tullus K, Verrier-Jones K, Lakhanpaul M.
Antibiotic prophylaxis for children at risk of
developing urinary tract infection: a systematic
review. Acta Paediatr 2009; 98: 1781-1786.
10. Williams G, Craig JC.
Prevention of recurrent urinary tract infection in
children. Curr Opin Infect Dis 2009; 22: 72-76.
11. Hoberman A, Keren R.
Antimicrobial prophylaxis for urinary tract
infection in children. New Engl J Med 2009; 361:
1804-1806.
12. Mattoo TK. Are prophylactic
antibiotics indicated after a urinary tract
infection? Curr Opin Pediatr 2009; 21: 203-206.
13. De Cunto A, Pennesi M, Salierno
P. Antibiotic prophylaxis for the prevention of
recurrent urinary tract infection in children with
low grade vesicoureteral reflux: Results from a
prospective randomized study. J Urol 2008; 180:
2258-2259.
14. Keren R, Carpenter M,
Greenfield S, Hoberman A, Mathews R, Mattoo T, et
al. Is antibiotic prophylaxis in children with
vesicoureteral reflux effective in preventing
pyelonephritis and renal scars? A randomized,
controlled trial. Pediatrics 2008; 122: 1409-1410.
15. Stansfeld JM. Duration of
treatment for urinary tract infections in children.
BMJ 1975; 3: 65-66.
16. Smellie JM, Katz G, Gruneberg
RN. Controlled trial of prophylactic treatment in
childhood urinary tract infection. Lancet 1978; 2:
175-178.
17. Savage DCL, Howie G, Adler K,
Wilson MI. Controlled trial of therapy in covert
bacteriuria of childhood. Lancet 1975; 1: 58-61.
18. Lohr JA, Nunley DH, Howards
SS, Ford RF. Prevention of recurrent urinary tract
infections in girls. Pediatrics 1977; 59: 562-565
19. Reddy P, Evans MT, Hughes PA,
Dangman B, Cooper J, Lepow ML, et al.
Antimicrobial prophylaxis in children with
vesico-ureteral reflux: a randomized study of
continuous therapy vs intermittent therapy vs
surveillance. Pediatrics 1997; 100 Suppl 3: 555-556.
20. Keren R, Carpenter MA, Hoberman
A, Shaikh N, Matoo TK, Chesney RW, et al.
Rationale and design issues of the Randomized
Intervention for Children with Vesicoureteral Reflux
(RIVUR) study. Pediatrics 2008; 122 Suppl 5:
S240-250.
21. Craig JC, Simpson JM, Williams
GJ, Lowe A, Reynolds GJ, McTaggart SJ, et al.
Antibiotic prophylaxis and recurrent urinary tract
infection in children. Prevention of Recurrent
Urinary Tract Infection in Children with
Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT)
Investigators. N Engl J Med 2009; 361: 1748-1759.
22. Roussey-Kesler G, Gadjos V,
Idres N, Horen B, Ichay L, Leclair MD, et al.
Antibiotic prophylaxis for the prevention of
recurrent urinary tract infection in children with
low grade vesicoureteral reflux: results from a
prospective randomized study. J Urol 2008; 179:
674-679.
23. Montini G, Rigon L, Zucchetta
P, Fregonese F, Toffolo A, Gobber D, et al.
Prophylaxis after first febrile urinary tract
infection in children? A multicenter, randomized,
controlled, noninferiority trial. Pediatrics 2008;
122: 1064-1071.
24. Pennesi M, Travan L,
Peratoner L, Bordugo A, Cattaneo A, Ronfani L, et
al. Is antibiotic prophylaxis in children with
vesicoureteral reflux effective in preventing
pyelonephritis and renal scars? A randomized,
controlled trial. Pediatrics 2008; 121: 1489-1494.
25. Garin EH, Olavarria F, Garcia
Nieto V, Valenciano B, Campos A, Young L. Clinical
significance of primary vesicoureteral reflux and
urinary antibiotic prophylaxis after acute
pyelonephritis: a multicenter, randomized,
controlled study. Pediatrics 2006; 117: 626-632.
26. Greenfield SP, Ng M, Wan J.
Resolution rates of low grade vesicoureteral reflux
stratified by patient age at presentation. J Urol
1997; 157: 1410-1413.
27. Schwab CW Jr, Wu HY, Selman
H, Smith GH, Snyder HM 3rd, Canning DA. Spontaneous
resolution of vesicoureteral reflux: a 15-year
perspective. J Urol 2002; 168: 2594-2599.
|
|
|
 |
|

