|
|
|
Indian Pediatr 2016;53:
39-41 |
 |
Association Between
Metabolic Control and Lipid Parameters in Indian Children with
Type 1 Diabetes
|
|
Lavanya Parthasarathy, Shashi Chiplonkar, Vaman
Khadilkar and Anuradha Khadilkar
From Hirabai Cowasji Jehangir Medical Research
Institute, Jehangir Hospital, Pune, Maharashtra, India.
Correspondence to: Dr Anuradha Khadilkar, Hirabai
Cowasji Jehangir Medical Research Institute, Jehangir Hospital, 32
Sassoon Road, Pune.411 001, India.
Email:
[email protected]
Received: April 07, 2015;
Initial review: June 08, 2015;
Accepted: October 28, 2015.
|
Objectives: To compare lipid parameters between diabetics and
controls and to study association between metabolic control and lipid
profile.
Methods: Lipid profile and HbA1c were measured (n=80,
39 boys) in diabetic children [age 10.7(3.4) y] and 54 controls, tests
repeated after 1 year (in 45 diabetics).
Results: Diabetic children had higher mean (SD)
LDL-C [95.3(27.7) vs 84.5(26.4) mg/dL], lower HDL-C [48.2 (13.1)
vs 53.1(11.9) mg/dl]. Moderate physical activity (P=0.014)
protected against high LDL-C levels. HbA1c (P=0.00) predicted
total and LDL-C levels. At 1year, 63% showed reduced LDL-C with
improving HbA1c; 72% showed increased LDL-C with deteriorated HbA1c.
Conclusion: Improving metabolic control is
cardinal to reduce cardiometabolic risk; physical activity is
beneficial.
Keywords: Cardiometabolic risk, Hyperlipidermic, Metabolic
control, Prognosis.
|
|
I
mpaired lipid metabolism resulting from
uncontrolled hyperglycemia is implicated in cardiovascular complications
in diabetes. Deranged lipids have been reported amongst adolescents and
youth in the SEARCH for Diabetes in Youth study [1]. Although data on
the influence of blood glucose control on development of atherosclerosis
is conflicting, there is increasing evidence of an association between
the two [2-5].
This study was conducted to i) compare lipid
parameters between diabetic children and controls; ii) determine
factors influencing lipid parameters in diabetic children; and iii)
examine effect of lowering of glycosylated hemoglobin on lipids in
diabetic children at one year.
Methods
This cross-sectional study with one-year follow-up
was done after institutional ethical committee clearance. Considering
variability in LDL-C reported in past studies [6], a sample size of 80
diabetics and 54 controls was found to have a power of 0.8 at 5% level
of significance and 3% margin of error. Families with diabetic children
aged 5 to 17 years attending the diabetes clinic at our institution were
approached, and a random sample of 80 (39 boys) were enrolled
prospectively (May 2013 to October 2014). Age- and gender-matched
healthy controls were recruited from private schools. Patients with
known chronic disorders were excluded. Data on medications, age at onset
and duration of diabetes and insulin regimen were collected in a
standardized form and verified from hospital records.
Anthropometric data were collected using standard
methods and were converted to Z scores [7]. Fasting blood sample was
collected to measure HbA1c by HPLC (BIO-RAD, Germany) and lipid profile
(enzymatic method). The GE-Lunar DPX Pro (GE Healthcare, Wisconsin, USA)
was used to measure body composition. Dietary intakes were assessed by
24-hour recall on three random days (non-consecutive)/week, including
one holiday. Nutrient intakes were calculated (CDIET, Version 2, Xenios
Technologies, Pune, India). Physical activity data were collected using
validated activity questionnaires [8,9] adapted for Indian children.
Statistical analyses were carried out using SPSS for
Windows 12 (SPSS, Chicago, IL, USA). Anthropometry, biochemical
parameters and body composition were recorded in 45 (20 girls) children
who regularly attended the clinic at the end of one year.
Results
Table I summarizes the anthropometric, body
composition and biochemical parameters. There were no significant
differences between sexes for anthropometric and biochemical parameters
(P>0.05). Hence, further analysis was performed on pooled data.
TABLE I Anthropometry, Body Composition and Biochemical Parameters in Study Participants
|
Cases (n=80) |
Controls (n=54) |
|
Age (y) |
10.7 (3.4) |
11.7 (2.8) |
|
Height (cm) |
132.3 (18.1) |
144.7* (15.2) |
|
Weight (kg) |
28.9 (11.8) |
35.0* (11.2) |
|
BMI (kg/m2) |
15.6 (3.1) |
17.1 (5.8) |
|
Height for Age Z Score |
–0.9 (1.1) |
0* (0.9) |
|
Weight for Age Z Score |
–1 (0.9) |
–0.8 (3.4) |
|
BMI for Age Z Score |
–0.7 (0.8) |
–1.3 (5.7) |
|
Lean Mass% |
73.9 (9.0) |
70.7 (9.0) |
|
Total Body Fat% |
20.3 (9.1) |
26.5* (12) |
|
Android Fat% |
21.9 (10.3) |
29.1* (13.3) |
|
Gynoid Fat% |
33 (8.8) |
35.8 (9.9) |
|
Cholesterol (mg/dL) |
157.7 (33.5) |
153.5 (27.7) |
|
Triglyceride (mg/dL) |
71 (26.5) |
71.5 (30.5) |
|
HDL (mg/dL) |
48.2 (13.1) |
53.1* (11.9) |
|
VLDL |
14.3 (5.4) |
14.3 (6.1) |
|
LDL (mg/dL) |
95.3 (27.7) |
84.5* (26.4) |
|
Cholesterol/HDL ratio |
3.4 (0.8) |
3* (0.8) |
|
LDL/HDL ratio |
2.1 (0.7) |
1.7* (0.7) |
|
HbA1c% |
10 (2) |
- |
|
*significantly different between diabetic children and
controls (P<0.05); #significantly different between
HbA1c>median group and controls (P<0.05). |
35% diabetic children had high LDL-C, 18% had low
HDL-C and 2% had high triglycerides [10]. HDL-C was significantly lower
and LDL-C was significantly higher in diabetic children. Mean (SD) HbA1c
of diabetic children was 10 (2), signifying suboptimal blood glucose
control.
Using linear regression, metabolic control (HbA1c) (P=0.002)
was identified as positive predictor and age at diagnosis (P=0.012)
as a negative predictor of total cholesterol. For LDL-C, metabolic
control (P=0.001) and age at diagnosis (P=0.027) were
predictors. Total body fat % or android fat % did not affect lipid
profile. When diabetic children were divided into two groups according
to median HbA1c (9.7%) and then compared to controls, diabetic children
with HbA1c above median had significantly higher LDL-C and lower HDL-C
as compared to controls (Fig. 1).
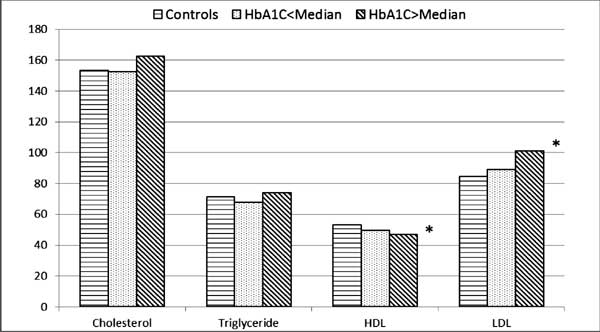 |
|
Fig. 1 Comparison of lipid
profile parameters between groups of diabetic children according
to HbA1C and controls.
|
To study impact of lifestyle factors on lipid
profile, linear regression models were utilized. Mean (SD) daily intake
of energy was 1632 (43) Kcal, protein was 44 (12) g, fat was 45 (12) g
and carbohydrate was 267 (75) g. Mean (SD) time spent in moderate
activities by children was 37 (25) min/d. Moderate physical activity (P=0.014)
was identified as protective factor against LDL-C. Dietary intakes did
not affect any of the lipid profile parameters.
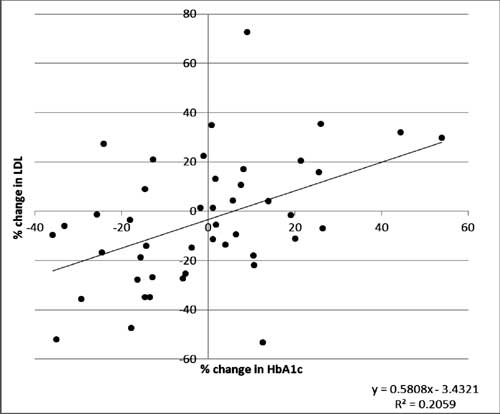
Follow-up: Lipid profile parameters and HbA1c
were repeated after one year in a subset of diabetic children (n=45)
and percentage change of LDL-C and HbA1c were computed. A positive
association (r 2=0.2059)
between percentage change in LDL-C and HbA1c was seen (Fig. 2).
In the group of children whose metabolic control improved, 63% children
showed a reduction in LDL-C levels. Whereas, in the group where the
metabolic control deteriorated, 72% children showed increase in LDL-C.
 |
|
Fig. 2 Relationship between
percentage change of LDL-C and HbA1c over the one year period.
|
Discussion
Our cross-sectional data shows that Indian children
and adolescents with type 1diabetes have deranged lipids as compared to
healthy controls. Deterioration was related to poor metabolic control
and age at diagnosis. Physical activity was protective against LDL-C.
One year follow-up data showed deterioration in HbA1c to be associated
with an increase in LDL-C and vice versa.
One of the important factors contributing to
variations in lipid profile amongst children could be family history of
the child. We did not have information regarding the parent’s lipid
profile parameters or their risk for CVD. Hence, we could not adjust for
this during analysis. Although our data support the relationship between
poor metabolic control and increase in LDL-C, our sample size for the
follow-up study is small, and studies with larger sample size are
required to confirm our findings.
Interference in normal physiology of low-density
lipoprotein (LDL) particle has been reported by studies in the past
which suggest that in patients with type 1 diabetes, LDLs are often
enriched in triglycerides and increased number of small dense LDL
particles are observed. Our data are in keeping with findings of Guy,
et al. [2] and Petitti, et al. [5] who have also reported
high LDL-C among children with Type 1 diabetes.
Advantages of physical activity in diabetes
management have been previously reported [11-13]. In our study as well,
children who engaged in moderate physical activity for more than half an
hour each day had lower LDL-C. Hence, encouraging children to
participate in various forms of physical activity may be beneficial in
reducing the risk of CVD in future.
In conclusion encouraging a healthy lifestyle
with adequate physical activity and improving metabolic control is
recommended for reducing dyslipidemia in children with diabetes.
Children diagnosed younger are more likely to have deranged lipids and
thus are at increased cardiometabolic risk.
Contributors: LP, AK, VK: conceptualized the
study, and analyzed data; SC: performed statistical analyses. All
authors contributed to the manuscript and approved its contents.
Funding: Mr. Pancharatnam; Ms Lavanya
Parthasarathy was funded by a fellowship grant from the University
Grants Commission, Government of India.
Competing interests: None stated.
|
What This Study Adds?
• Children diagnosed at younger years are at
higher risk of deranged lipid parameters.
• Physical activity is a protective factor against desanyed
lipid profile.
|
References
1. Kershnar AK, Daniels SR, Imperatore G, Palla SL,
Petitti DB, Pettitt DJ, et al. Lipid abnormalities are prevalent
in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in
Youth study. J Pediatr. 2006;149:314-9.
2. Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis
EJ, Liese AD, et al. Lipid and lipoprotein profiles in youth with
and without type 1 diabetes: the SEARCH for Diabetes in Youth
case-control study. Diabetes Care. 2009;32:416-20.
3. Ladeia AM, Adan L, Couto-Silva AC, Hiltner A,
Guimarães AC. Lipid profile correlates with glycemic control in young
patients with type 1 diabetes mellitus.Prev Cardiol. 2006;9:82-8.
4. Petitti DB, Imperatore G, Palla SL, Daniels SR,
Dolan LM, Kershnar AK, et al. SEARCH for Diabetes in Youth Study
Group. Serum lipids and glucose control: the SEARCH for Diabetes in
Youth study. Arch Pediatr Adolesc Med. 2007;161:159-65.
5. Schwab KO, Doerfer J, Naeke A, Rohrer T, Wiemann
D, Marg W, et al. ; German/Austrian Pediatric DPV Initiative.
Influence of food intake, age, gender, HbA1c, and BMI levels on plasma
cholesterol in 29,979 children and adolescents with type 1
diabetes—reference data from the German diabetes documentation and
quality management system (DPV). Pediatr Diabetes. 2009;10:184-92.
6. Babar GS, Zidan H, Widlansky ME, Das E, Hoffmann
RG, Daoud M, et al. Impaired endothelial function in
preadolescent children with type 1 diabetes. Diabetes Care.
2011;34:681-5.
7. Khadilkar VV, Khadilkar AV, Cole TJ, Sayyad MG.
Cross-sectional growth curves for height, weight and body mass index for
affluent Indian children, 2007. Indian Pediatr. 2009;46:477-89.
8. Barbosa N, Sanchez CE, Vera JA, Perez W, Thalabard
JC, Rieu M. A physical activity questionnaire: Reproducibility and
validity. J Sports Sci Med. 2007;6:505-18.
9. CDC, 1999. Available from: www.cdc.gov/nccdphp/
dnpa/ physical/pdf/PA_Intensity_table_2_1.pdf. Accessed December 22,
2013.
10. American Diabetes Association. Management of
dyslipidemia in children and adolescents with diabetes. Diabetes Care.
2003;26:2194-7.
11. Beraki A, Magnuson A, Särnblad S, Åman J,
Samuelsson U. Increase in physical activity is associated with lower
HbA1c levels in children and adolescents with type 1 diabetes: results
from a cross-sectional study based on the Swedish Pediatric Diabetes
Quality Registry (SWEDIABKIDS). Diabetes Res Clin Pract.
2014;105:119-25.
12. Quirk H, Blake H, Tennyson R, Randell TL,
Glazebrook C. Physical activity interventions in children and young
people with Type 1 diabetes mellitus: a systematic review with
meta-analysis. Diabet Med. 2014;31:1163-73.
13. Leclair E, de Kerdanet M, Riddell M, Heyman E.
Type 1 diabetes and physical activity in children and adolescents. J
Diabetes Metab. 2013;S10:004.
|
|
|
 |
|

