|
|
|
Indian Pediatr 2014;51:
27-31 |
 |
Prevalence of Rotavirus Diarrhea among
Hospitalized Under-five Children
|
|
MA Mathew, Abraham Paulose, S Chitralekha, *MKC Nair,
†Gagandeep Kang and
‡Paul Kilgore
From Department of Pediatrics, Malankara Orthodox Syrian Church
Medical College Hospital, Kolenchery, Ernakulam District, Kerala; †Department
of Microbiology, Christian Medical College, Vellore, India and ‡Division
of Translational Research, International Vaccine Institute, Seoul, South
Korea.
Correspondence to: Dr MA Mathew, Professor, Department of Pediatrics,
Malankara Orthodox Syrian Church Medical College Hospital, Kolenchery,
Ernakulam District, Kerala 682 311, India.
Email: [email protected]
Received: February 02, 2013;
Initial review: March 05, 2013;
Accepted: August 22, 2013.
Published online: September 05, 2013.
PII: S097475591300111
|
Objectives: To estimate the prevalence of rotavirus diarrhea among
hospitalized children less than 5 years of age in Kerala State and to
determine the circulating strains of rotavirus in Kerala.
Design: Multicenter, cross-sectional study.
Setting: Eight representative hospitals in
Kunnathunadu Thaluk, Ernakulam district, Kerala.
Participants: Children in the age group under 5
years
Methods: Hospitalized children admitted with
acute diarrhea were examined and standardized case report form was used
to collect demographic, clinical and health outcome. Stool specimens
were collected and ELISA testing was done. ELISA rotavirus positive
samples were tested by reverse transcription PCR for G and P typing (CMC
Vellore).
Results: Among the 1827 children, 648 (35.9%)
were positive for rotavirus by the Rotaclone ELISA test. The prevalence
of rotavirus diarrhea in infants less than 6 months of age was 24.7%; 6-
11 months 31.9%; 12- 23 months 41.9%; 24- 35 months 46.9%; and 33.3% in
36- 59 months. Rotavirus infections were most common during the dry
months from January through May. G1P[8] (49.7%) was the most common
strain identified followed by G9P[8] (26.4%), G2P[4] (5.5%), G9P[4]
(2.6%) and G12P[6] (1.3%).
Conclusions: The prevalence of rotavirus diarrhea
among hospitalized children less than 5 years is high in Ernakulam
district, Kerala State.
Keywords: Kerala, Rotavirus diarrhea, Rotavirus infections.
|
|
R
otavirus is a leading cause of severe acute
gastroenteritis requiring hospitalization among infants and young
children worldwide [1]. Data on rotavirus disease burden are needed
across India to support credible, evidence-based decisions regarding any
intervention. There is a lack of nationally representative data on the
incidence of severe rotavirus disease in India [2]. Previous studies in
the Indian Rotavirus Strain Surveillance Network have confirmed that
rotavirus accounts for 39% of acute diarrheal hospitalizations [3].
There is a need for additional research and public health surveillance
to ensure that adequate information about rotavirus is obtained from
diverse populations in India.
There are limited data on rotavirus disease burden
among children in Kerala. We conducted a systematic study of rotavirus
diarrhea among children in Ernakulam district, Kerala with the
objectives to estimate the prevalence of diarrhea due to rotavirus among
hospitalized children younger than 5 years of age and also to describe
the circulating strains of rotavirus in this population.
Methods
This was a multicenter study conducted in 8 hospitals
in Kunnathunadu Thaluk, Ernakulam district, Kerala. In 2001 national
census, Kunnathunadu had 47,743 children less than 5 years of age. For
this study the Malankara Orthodox Syrian Church Medical College (MOSC),
a tertiary care referral hospital, was the base hospital and 2
government and 5 private hospitals were selected.
All children aged <5 years who presented to a study
hospital with acute watery diarrhea and required hospitalization were
enrolled after informed consent was obtained from the parent or
guardian. The study was conducted over a period of 24 months between
February 1, 2009 and January 31, 2011.
A case of diarrhea was defined as increased stool
frequency compared with the usual pattern occurring in a child less than
5 years old for whom parents sought care for treatment of diarrhea. The
indications for hospitalization were (i) severe dehydration
requiring intravenous hydration, (ii) malnourished children with
dehydration, (iii) toxic appearance, changing mental status or
seizure, (iv) fever >38.5°C for infants <6 months or >39°C for 6-
36 months, (v) high output diarrhea ( >10 large volume
stool/day), (vi) persistent vomiting or diminished or no oral
intake, (vii) suboptimal or no response to ORT or further
deterioration, (viii) inability of the caregiver to administer
ORS, (ix) suspected surgical cause, and (x) history of
premature birth, chronic medical conditions or concurrent illness [4,5].
Hospitalized children less than 5 years of age
admitted with acute diarrhea were examined by trained medical staff. The
subject’s parent or guardian was interviewed concerning date of onset of
diarrhea and on vomiting and fever. Information was collected on the
duration of diarrhea, maximum number of stools passed per day, duration
and frequency of vomiting, degree of fever, presence and severity of
dehydration, and treatment. The severity of dehydration was assessed
according to the WHO Integrated Management of Childhood Illness Model
Handbook guidelines and was categorized into severe, some or no
dehydration [5]. Data concerning primary method of feeding and duration
of exclusive breast feeding were collected.
A standardized case report form based on the WHO
generic protocol [6] was used to collect demographic, clinical and
health outcome data.
Stool collection and laboratory analysis: Stool
specimens were collected from hospitalized patients and stored in the
refrigerator at 4°C and later transported to the base hospital in icebox
and stored at -20°C in the testing laboratory. ELISA testing (RotaClone,
Meridian diagnostics, Cincinnati,OH) was done twice weekly for detection
of rotavirus antigen. The enzyme-linked immunosorbent assay was highly
sensitive (100%) and specific (97%) for rotavirus antigen. ELISA
Rotavirus-positive samples were analyzed by reverse
transcription-polymerase chain reaction for G and P typing at Christian
Medical College, by previously reported methods [7].
Data management and statistical analysis: Data
were entered on a weekly basis into database management software, which
was created for the surveillance system and was based on an MS Visual
FoxPro platform. Analysis was performed using SAS software. Tests of
proportion were applied. A P value <0.05 was considered to be
statistically significant.
The protocol was reviewed and approved by the local
independent ethics committee/ institutional review board for each
participating centre.
Results
1827 children with diarrhea were admitted to study
health facilities and had a stool specimen collected for rotavirus
testing during the study period. There were 20 children just above five
years of age; they were excluded from final analysis (n=1807). Of
the 1807 stool specimens tested, 648 (35.8%) were positive for rotavirus
by the RotaClone ELISA test (Table I). The mean (SD) age
of children was 17.9 (13.8) months; male 17.2 (13.7) vs. female
18.8 (13.9).
TABLE I Rotavirus Results of Children With Diarrhea, Kerala, India, January 2009 to January 2011
|
RV-Positive (n= 648)
|
|
Age group (mo) |
|
|
<6 (n=235) |
58 (24.7%)
|
|
6-11 (n=568) |
181 (31.9%)
|
|
12-23 (n=535) |
233 (41.9%)
|
|
24-35 (n=194) |
91 (46.9%)
|
|
36-59 (n=255) |
85 (33.3%)
|
|
Fever group (°C)* |
|
|
≤ 37.5 (n=935) |
307 (32.8%)
|
|
37.6 -38.6 (n=690) |
256 (37.1%)
|
|
≥ 38.7 (n=182) |
85 (46.7%)
|
|
Length of stay (days)* |
|
|
≤ 2 (n=858) |
300 (34.9%)
|
|
3- 6 (n=779) |
267 (34.3%)
|
|
≥ 7 (n=170) |
81 (47.6%)
|
|
Dehydration# |
|
|
None (n =1292)
|
416 (32.2%)
|
|
Some (n =503) |
228 (45.3%)
|
|
Severe (n =9) |
4 (44.4%)
|
|
*P<0.01; #P<0.001. |
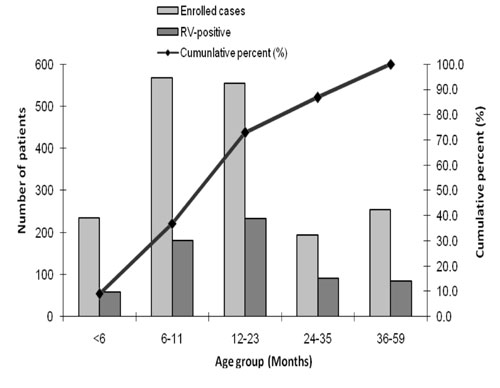 |
|
Fig. 1 Age distribution of children
with diarrhea, Kerala, India, February 2009 to January 2011.
|
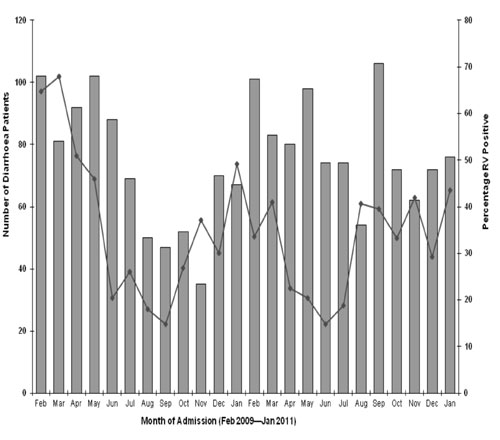 |
|
Fig. 2 Monthly distribution of
rotavirus-positive patients, Kerala, India, February 2009 –
January 2011 (n= 1807).
|
Fig. 1 shows the cumulative age distribution
of rotavirus cases. There was no mortality in the study population.
Rotavirus infections were seen throughout the year and were most common
during the hot dry months from January through May (Fig. 2).
Of the 648 samples that were ELISA rotavirus positive, genotyping (Table
II) was done for 450 (81.6%) randomly selected samples. The majority
(49.7%) of rotavirus strains typed were G1P[8] strains. An additional 12
(2.6%) samples were untypable.
TABLE II Distribution Of G And P Types Among A Randomly Selected Subset (N=450)
Of Rotavirus Positive Samples
Genotype
(n=450) |
Number
|
|
G1 P[8] |
224 ( 49.7%) |
|
G9 P[8] |
119 (26.4 %) |
|
G2 P[4] |
25 (5.5%)
|
|
G9 P[4] |
12 (2.6%) |
|
G12 P[6] |
6 (1.3%) |
|
G1 P[6] |
4 (0.8%) |
|
G12 P[8] |
4 (0.8%) |
|
G1 P[4] |
1 (0.2%) |
|
G1 P[Untypable] |
2 (0.4%) |
|
G9 P[Untypable] |
11 (2.4%) |
|
Partially typed |
6 (1.3%) |
|
Mixed infections |
24 (5.3%) |
|
Both G and P untypable |
12 (2.6%) |
Discussion
This is the first systematic study to assess the
prevalence of rotavirus diarrhea among children younger than 5 years of
age in Kerala. In this study, rotavirus was detected in 35.9% of
diarrhea-related hospital admissions among children less than 5 years of
age in Ernakulam district, Kerala.
A review of studies performed in India during 1990-
2005 had estimated that rotavirus disease accounted for 20.8% of all
diarrhea- related hospital admissions [7]. The Indian Rotavirus Strain
Surveillance Network carried out a multi-centric study in seven
different regions of India and reported that rotavirus was detected in
stools of 39% children aged <5 years [3]. Inclusion of children at
hospitals caring for lower acuity diarrheal episodes or less severe
disease may account for a lower percentage of rotavirus positive cases
among the total number of enrolled patients in our study compared with
previous studies [3,8-15].
The prevalence of rotavirus diarrhea in infants aged
<6 months was 24.7%, with high prevalence in children aged 6 months to
11 months and 12-23 months (31.9% and 41.9%, respectively). These data
are important because they demonstrate that the vast majority of cases
could be prevented by an effective rotavirus vaccine given to children
along with their primary immunization series. But for a setting in a
developing country, this is somewhat lower than expected since ~80% to
85% of all rotavirus cases in children under 5 years occurred by 18
months of age in hospital-based studies [16]. These observations may
indicate that the epidemiology of rotavirus in Kerala may differ from
other parts of India, because general living conditions and
socio-economic status are better in Kerala.
A marked seasonality was not seen in our study and
rotavirus infections peaked from January through May and were less
common during the monsoon season months of June through September.
Rotavirus is markedly seasonal in Northern temperate locations but was
less seasonal in Southern locations with a tropical climate
[3,10,17,18]. It has been observed that with minimal seasonality,
rotaviruses circulate at a relatively higher level all year round,
resulting in children being exposed at an early age and experiencing
severe illness [14].
This is the first study in Kerala to provide
information on both rotavirus G and P types. The results of G and P
typing shows that the G1P[8] strain is the most common contributing half
the number of cases. This finding is also consistent with the results
from national rotavirus surveillance in India showing that the G1P[8]
strain was among the two most common strains from December 2005 to
November 2007. However, there were differences in that the G9P[8] strain
is the second most common strain found in the Kerala field site and 5.5%
of strains were G2P[4], while overall in the national Indian
surveillance network the G2P[4] and G9 P[8] strains accounted for 25.7%
and 8.5%, respectively of all rotavirus strains [3]. A recent study from
Vellore reported that the most common types were G1P[8] (in 15.9% of
infections), G2P[4] (in 13.6%), G10P[11] (in 8.7%), G9P[8] (in 7.2%),
G1P[4] (in 4.4%), G10P[4] (in 1.7%), G9P[4] (in 1.5%), G12P[6] (in
1.1%), and G1P[6] (in 0.6%) [19]. The proportion of untypable strains
may suggest the potential for emergence of new rotavirus strains in
Kerala.
The strengths of this study include the use of the
WHO generic protocol and laboratory confirmation of rotavirus diarrhea
in a single reference laboratory including genotyping of rotavirus
strains. Potential limitations are the small study population and the
lack of ability to extrapolate disease burden to milder disease because
this was a hospital-based study.
In conclusion, this study highlights that rotavirus
diarrhea accounts for a large proportion of diarrheal disease in
hospitalized children less than 5 years in Ernakulam district in Kerala.
Contributors: MAM: conceived and designed the
study and revised the manuscript for important intellectual content. He
will act as guarantor of the study; AP: collected data and drafted the
paper; MKC, GK and SC: did final revision of manuscript. GK conducted
the laboratory tests, and interpreted them; PK: analysed the data and
helped in manuscript writing. The final manuscript was approved by all
authors.
Funding: Indian Council for Medical Research, New
Delhi; Competing interests: None stated.
|
What Is Already Known?
•
Rotavirus is the leading cause of severe diarrhea in Indian
children less than 5 years.
What This Study Adds?
• High prevalence of rotaviral diarrhea in
Ernakulam, Kerala state, accounting for 35.9% of diarrhea-related
hospitalizations among children less than 5 years of age.
|
References
1. Centers for Disease Control and Prevention (CDC).
Rotavirus surveillance- worldwide, 2001- 2008. MMWR Morb Mortal Wkly
Rep. 2008;57:1255-7.
2. Khan G, Fitzwater S, Tate JE, Kang G, Ganguly N,
Nair G, et al. Epidemiology and prospects for prevention of rotavirus
disease in India. Indian Pediatr. 2012;49:467-74.
3. Kang G, Arora R, Chitambar SD, Deshpande J, Gupte
MD, Kulkarni M, et al. Multicenter, hospital-based surveillance of
rotavirus disease and strains among Indian children aged <5 years. J
Infect Dis. 2009;200:S147-53.
4. Bhatnagar S, Lodha R, Choudhary P, Sachdev HP,
Shah N, Narayan S, et al. Consensus statement of IAP National Task
Force: Status report on management of acute diarrhea. Indian Pediatr.
2007;44: 380-9.
5. World Health Organization. The Treatment of
Diarrhea: A Manual for Physicians and Other Senior Health Workers, 4th
ed. Geneva: World Health Organization, 2005
(http://www.who.int/child-adolescent health/New_Publications/CHILD_HEALTH/ISBN_92_4_159318_0.pdf).
Accessed on May 12, 2013.
6. Bresee J, Parashar U, Holman R, Gentsch J, Glass
R. Generic protocols for (i) hospital-based surveillance to estimate the
burden of rotavirus gastroenteritis in children and (ii) a
community-based survey on utilization of health care services for
gastroenteritis in children. Document WHO/V&B/02.15.Geneva: World Health
Organization, 2002:1-67.
7. Ramani S, Kang G. Burden of disease and molecular
epidemiology of group A rotavirus infections in India. Indian J Med Res.
2007;125:619-32.
8. Samajdar S, Ghosh S, Chawla- Sarkar M, Mitra U,
Dutta P, Kobayashi N, et al. Increase in prevalence of human group A
rotavirus G9 strains as an important VP7 genotype among children in
eastern India. J Clin Virol. 2008;43:334-9.
9. Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M,
Parashar U, et al. Incidence of severe rotavirus diarrhoea in New Delhi,
India, and G and P types of the infecting rotavirus strains. J Infect
Dis. 2005;192:S114-9.
10. Saravanan P, Ananthan S, Ananthasubramanian M.
Rotavirus infection among infants and young children in Chennai, South
India. Indian J Med Microbiol. 2004;22:212-21.
11. Kang G, Green J, Gallimore CI, Brown DW.
Molecular epidemiology of rotaviral infection in South Indian children
with acute diarrhoea from 1995-1996 to 1998-1999. J Med Virol.
2002;67:101-5.
12. Banerjee I, Ramani S, Primrose B, Moses P,
Iturriza- Gomera M, Gray JJ, et al. Comparative study of the
epidemiology of rotavirus in children from a community-based cohort and
a hospital in South India. J Clin Microbiol. 2006;44:2468-74.
13. Kelkar Sd, Purohit SG, Simha KV. Prevalence of
rotavirus diarrhoea among hospitalized children in Pune, India. Indian J
Med Res.1999:109:131-5.
14. Mishra V, Awasthi S, Nag VL, Tandon R. Genomic
diversity of group A rotavirus strains in patients aged 1-36 months
admitted for acute watery diarrhoea in northern India: a hospital based
study. Clin Microbiol Infect. 2010;16;45-50.
15. Chakravarti A, Chauhan MS, Sharma A, Verma V.
Distribution of human rotavirus G and P genotypes in a hospital setting
from Northern India. Southeast Asian J Trop Med Public Health.
2010;41:1145-52.
16. Nelson EA, Bresee JS, Parashar UD, Widdowson MA,
Glass RI. Rotavirus epidemiology: the Asian Rotavirus Surveillance
Network. Vaccine. 2008;26:3192-6.
17. Ray P, Sharma S, Agarwal RK, Longmei K, Gentsch
Jr, Paul VK, et al. First detection of G12 rotavirus in newborns with
neonatal rotavirus infection at All India Institute of Medical Sciences,
New Delhi, India. J Clin Microbiol. 2007;45:3824-7.
18. Phukan AC, Patgiri DK, Mahanta J. Rotavirus
associated acute diarrhoea in hospitalized children in Dibrugarh, Nort-east
India. Indian J Pathol Microbiol. 2003;46:274-8.
19. Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J,
Sarkar R, Rehman AM, et al. Protective effect of natural rotavirus
infection in an Indian birth cohort. N Engl J Med. 2011;365:337-46.
|
|
|
 |
|

