I
n-hospital cardiac arrest has high mortality and
significant neurologic morbidity. Despite resuscitation measures, death
rates for such patients remain high and only 17% of adults and 27% of
children survive to hospital discharge [1,2]. Patients admitted from
hospital wards to Critical Care Unit (CCU) have higher mortality than
patients admitted from emergency department [3]. It is well established
that physiological abnormalities exist before cardiac arrest [4]. These
studies suggested that it may be possible to develop strategies to
prevent cardiac arrest in hospitalized patients.
Implementation of Rapid Response Systems (RRS) is
believed to improve efficacy in recognizing and responding to
deteriorating patients. These have been known as Medical Emergency Team
(MET), Rapid Response Team or Outreach Team. METs include at least one
critical care physician while RRTs can be led by nurses or respiratory
therapists. Since 1990 when they were first described in Australia, RRS
have been effective in reducing hospital mortality, CCU admissions and
arrest before transfer to CCU. Many hospitals have implemented them
across North America. These teams are similar in that they rely on
prompt identification and treatment of in-hospital patients [5]. The
Institute for Healthcare Improvement (IHI) included deployment of RRT as
one of the main recommendations in their 100,000 Lives Saved and the 5
Million Lives Saved campaigns [6]. RRS is different from Code team in
that they assess patients in whom respiratory, neurologic or cardiac
deterioration develops rather than patients who already had a
respiratory or cardiac arrest [7]. In some models one team have both
functions.
Characteristics of Rapid Response Systems
There are four components in any RRS: (1) the
afferent limb identifies deterioration in the patient and triggers a
response (consists of calling criteria for activating the RRS plus the
personnel who can trigger system activation); (2) the efferent limb
consists of the personnel (and equipment) brought to the patient; (3)
the audit or monitoring component focuses on patient safety and quality
improvement and provides feedback and evaluation of the events to the
providers, healthcare system designers and to the patient and families;
(4) the governance or administrative component which ensures ongoing
training and education of staff, implementation and sustaining the
service [8]. The composition of RRS team depends on the institutional
resources and goals, and the purposes of the team.
The objectives of having a MET at our Hospital were:
(i) As a tool to strengthen the culture of patient safety
throughout the institution by taking the critical care skills to all
corners of the hospital; (ii) to reduce the code blue events; (iii)
to reduce the unplanned admissions to the Critical care unit; (iv)
to reduce the readmission rates to the Critical care unit; and (v)
to educate the medical and nursing staff on the floors on issues related
to recognition and initial management of critically ill children.
Blueprint for Establishing a Rapid Response System
Establishing a RRS is a process that should be guided
by local needs and availability of personnel and resources. The first
step is to demonstrate a need, and then buy-in from the hospital
managers and clinical services. You may need to spend up to six months
or longer to complete a needs assessment, build a case for a RRS, and
develop a model that would best suit your institution.
Barriers to Employment of RRS
One of the most important goals of the MET in our
institution has been "empowerment" of all members of the
interprofessional healthcare team to seek help in stabilizing and
managing the deteriorating ward patient. Traditional institutional
hierarchy can be a serious barrier to this cultural change.
Wholehearted acceptance of a MET at all levels of a
hospital may take years and requires repeated and continuous education
and periodic satisfaction surveys. These periodic surveys will help to
identify areas that the MET should improve upon and also obstacles to
the utilization of the service. Strategies we used to overcome the
apparent and potential barriers were:
For six months, we went to essentially every
stakeholder group in the hospital (clinicians, managers) and gave
presentations about the team and answered the questions and concerns
raised.
We asked the callers to always inform the most
responsible physician at the same time they requested a MET consult.
We included the pediatric residents in our team
to increase collaboration and address concerns about de-skilling of
the residents.
|
Box 1. Blueprint for Establishing a Rapid
Response System
The RRS should be planned and tailored
locally.
Needs assessment: Review the charts of Code
Blue patients in your institution, urgent admissions to the CCU,
and readmissions to the CCU.
Establish who the members of the Team are
and what their skill levels should be.
Establish an RRT or MET structure that
would best suit your hospital.
Establish criteria on when to activate the
RRS.
Create data collection, documentation, and
record keeping tools.
Provide hospital-wide education prior to
rolling out.
Start as a pilot project with your best
people and best coverage during the day-time.
Audit; develop feedback and evaluation
mechanisms
Full 24 x 7 rolling out phase
Provide initial and ongoing education for
responders
Assess satisfaction rate and collect feedback from the
stakeholders.
|
Effect on Patient Outcome
Many single center studies [9-14] have demonstrated
effectiveness of the RRS. The only pediatric multicenter study to
determine the effectiveness of a RRS showed that there is a decrease in
rate of CCU mortality after readmission but not actual cardiopulmonary
arrest rate [9]. Decisions about whether to implement RRS will rely on
the individual institution.
Hospital for Sick Children Experience
In March 2006, four Pediatric Academic Health Science
Centres in Ontario were granted funding by the Ministry of Health and
Long-Term Care to initiate pediatric METs. These centres worked together
to develop and implement a pediatric MET in their respective institution
in a standardized method across the four sites. Each centre was tasked
with developing and implementing team that best met the needs of their
individual institution. The method has been described elsewhere [15].
The program was introduced in three phases at the Hospital for Sick
Children in Toronto.
Phase 1: (May to October 2006): Planning and
development of the core requirements for the team: A hospital-wide
algorithm for activating the MET, as well as the calling criteria was
agreed upon. Team make-up and roles were defined, and the hiring process
was initiated. Concurrently, a broad public relations plan was
implemented. The public relations strategy spanned six months with over
150 presentations given to the clinical interprofessional groups
(physicians, nursing, physio-therapy, respiratory therapy, social work)
and hospital management.
Phase 2: (November 2006 to January 2007): MET
service was introduced on a limited basis Monday to Friday, 08:00
to16:00. This allowed the team to ramp up their internal educational
needs, as well as to begin the integration of the MET into the hospital
environment. All team members attended simulation-based courses focusing
on the identification, assessment and management of the deteriorating
pediatric patient. At the same time, an education curriculum was
developed that met the educational needs of ward/clinic staff. The
public relations presentations continued throughout Phase 2 in order to
maintain momentum and answer any questions that might have arisen during
implementation.
Phase 3: (February 2007-ongoing): Full
24/7 service was rolled out to all areas of the hospital. Further
refinement on the roles occurred based on feedback received from
satisfaction surveys. Data collection on key outcome measures also
commenced for every new consult (The reason for activation; who called
the team; how long the activation criteria were present before calling
the team; the primary service, the time to response by the team,
recommendations and treatments initiated by the team, the outcome of the
consult) and for all follow-ups post new consults and post-discharge
from the CCU.
Educational Footprint
At our hospital another equally important MET mandate
is provision of both formal and informal educational opportunities for
MET members as well as for ward staff (nurses, allied health workers,
and residents). To this extent, the team developed educational programs
to meet the unique needs of the staff. Forums for delivery include lunch
& learn sessions, monthly rounds/meetings, hour-long in-services, and a
twice yearly full day simulation based education session. Also, there
has been formal integration of pediatric residents into team activities
in order to address any concerns about minimizing educational
opportunities and "de-skilling" of the residents. The "MET rotation"
provides residents with a defined set of learning objectives that focus
on assessment and management of acute deterioration, and the management
of Code Blue. At the end of their rotation, a formal evaluation process
is conducted, with feedback from the MET MD lead and MET members.
Pre- and Post-Implementation Surveys
Pre-implementation surveys were distributed over
three months in order to get a sense of the existing culture in the
institution. The pre-implementation survey indicated a need for a
service that the staff could call to seek help and advice about rapidly
deteriorating patients. The Ontario Critical Care Secretariat performed
a post-implementation survey in January 2011. The questions reflected
the core functions of the teams and the open-ended questions allowed for
more comments. We found:
Over 92% of physician and nurse respondents had
participated in the care of a patient with the MET.
Most respondents (98%) agreed that the MET was
used primarily for consults for unstable patients on the ward.
Respondents identified that MET were used to
support end of life discussion and education and advice on drugs.
The majority of respondents (>95%) were satisfied
with both the quality and timeliness of the MET service. Also >90% of
respondents believed that the MET has had a positive impact on patient
care. When asked if there were barriers to calling the MET 23% answered
in the affirmative. Of these, over 70% identified "MET responds
negatively if they deem the call inappropriate"[16].
Toronto Hospital for Sick Children MET Calling
Criteria
Call MET if one or more of the following exists: (For
age specific criteria refer Table I).
Healthcare Provider worried
Airway threat (any concerns by the provider
that airway is compromised, i.e. noisy breathing, stridor, increased
work of breathing).
Saturation <90% in any amount of O
2;
saturation <60% in any amount of O2
in children with cyanotic heart disease.
Respiratory distress (any concern by the
provider that the frequency or work of breathing is abnormal, any
apneas).
Tachycardia, Bradycardia (as explained in
age-adjusted Table I).
Hypotension (as explained in age-adjusted table
I), poor peripheral pulses, prolonged capillary refill time, mottled
extremities.
Acute change in neurological status, decreased
activity or responsiveness in small infants, acute drop in GCS by
more than 2, Seizures.
TABLE I The Hospital for Sick Children MET Calling Criteria (Age-adjusted Physiological Parameters)
|
Age |
Hypotension |
Brady- |
Tachy- |
Tachypnea |
|
Systolic BP |
cardia |
cardia |
|
|
Term 3 mo |
<50 |
<100 |
>180 |
>60 |
|
4 12 mo |
<60 |
<100 |
>180 |
>50 |
|
1 4 yr |
<70 |
<90
|
>160 |
>40 |
|
5 12 yr |
<80 |
<80 |
>140 |
>30 |
|
>12 yr |
<90 |
<60 |
>130 |
>30 |
Review of the Activity of the MET at the Hospital for
Sick Children in Toronto in 2011
The following figures represent the activity of the
MET during 2011 at the Hospital for Sick Children in Toronto (2011 is
chosen as a representative year; our data from 2007 to 2012 show a
similar pattern) (Fig.1 and 2):
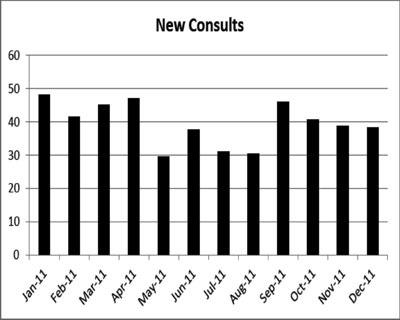 |
|
Fig. 1 The number of new consults
according to the month of the year.
|
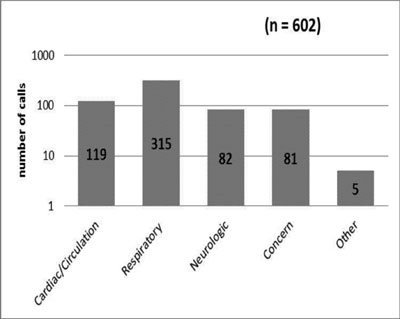 |
|
Fig. 2 Indication for the MET
consults.
|
Conclusion
In a survey done by the Ontario Ministry of Health
and Long-Term Care in 2011, three of the perceived benefits of the MET
at our Hospital were: (1) the education we provide on the hospital
floors and clinics, (2) the satisfaction of our users (patients, nurses,
and physicians), and (3) empowerment of the bedside staff [16]. Bedside
nurses provide direct and continuous care and are the first to recognize
a deteriorating patient. The physiological signs of deterioration may be
observed or recorded by bedside staff but frequently are not recognized
or acted upon in a timely manner [17]. Increasingly more patients with
significant residual pathology or decreased physiological reserve or
dependency on technology are discharged to the hospital wards. The
increasing acuity of patients and lack of resources and inadequate
educational support for the bedside staff on the wards might be
contributing factors to failure to rescue deteriorating patients
[18-20]. Rapid Response Systems have been implemented to prevent failure
to rescue events [21]. The goal of RRSs is to take the critical care
expertise and resources out of the CCU walls to all corners of a
hospital hoping that this would lead to a decrease in cardiopulmonary
arrest rate and unexpected CCU admission.
At the Hospital for Sick Children in Toronto, we have
not witnessed a significant reduction in the Code Blue rate or the
readmission rate to the CCU with implementation of the MET [9,22]. There
are many reasons that a Code Blue is called at our hospital; many a
times a Code is called to get help, medication, equipment, or skills at
the bedside in a timely manner. In the majority of patients who "coded"
on the wards in our hospital, the MET have not been involved prior to
the Code or have been involved for less than 4 hours prior to the Code.
25% of the readmissions to CCU occur within 6 hours of their first MET
visit post CCU discharge [22].
When comparing the readmission rates as well as the
outcome following readmission during the three eras (the two years
before MET, 2 early MET, and 2 mature MET years; span of 2005 to 2011)
there was no significant difference in the readmission rate [22].
It is likely that the causes for Code Blue or
readmission to the CCU after discharge require a different approach to
using the rapid response system at our hospital. We have seen a decrease
in mortality of the readmitted patients [9], which means these patients
were in a "better shape" when readmitted, or the input from the team has
stabilized them to some degree before readmission. The length of time to
readmission was reduced from 23.8 hours (12.3 32.7) to 17.9 hours (8.4
27.0) [22].
Before implementing a RRS, we need to ask what do we
want to achieve with the team and try to design the team to achieve
those goals. The efficiency and cost effectiveness of RRS in developing
countries is an open question that has not been answered yet, and it is
important for planners to consider their local needs and skills when
embarking upon implementing a RRS.
Acknowledgement: Dr Afrothite Kotsakis for
constructive criticism and review of the article.
Contributors: VK: conceived, designed and drafted
the initial manuscript; RG: helped in acquisition of data and writing
the manuscript; HMB: analyzed the data, revised and re-revised it
critically. All authors approved the final version.
Funding: None; Competing interests: None
stated.
References
1. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye
W, Mancini ME, et al. First documented rhythm and clinical
outcome from in-hospital cardiac arrest among children and adults. JAMA.
2006;295:50-7.
2. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni
V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in
the hospital: a report of 14720 cardiac arrests from the National
Registry of Cardiopulmonary Resuscitation. Resuscitation.
2003;58:297-308.
3. Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques
T, Norman SL, et al. Duration of life-threatening antecedents
prior to intensive care admission. Intensive Care Med. 2002;28:1629-34.
4. Young KD, Seidel JS. Pediatric cardiopulmonary
resuscitation: a collective review. Ann Emerg Med. 1999;33:195-205.
5. Jones DA, DeVita MA, Bellomo R. Rapid-response
teams. N Engl J Med. 2011;365:139-46.
6. Protecting 5 Million Lives from Harm, Institute of
HealthCare Improvement, Cambridge, MA, USA. Available from:
http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx.
Accessed on February 1, 2013.
7. Litvak E, Pronovost PJ. Rethinking rapid response
teams. JAMA. 2010;304:1375-6.
8. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi
A, Teres D, et al. Findings of the first consensus conference on
medical emergency teams. Crit Care Med. 2006;34:2463-78.
9. Kotsakis A, Lobos AT, Parshuram C, Gilleland J,
Gaiteiro R, Mohseni-Bod H, et al. Implementation of a multicenter
rapid response system in pediatric academic hospitals is effective.
Pediatrics. 2011;128:72-8.
10. Hanson CC, Randolph GD, Erickson JA, Mayer CM,
Bruckel JT, Harris BD, et al. A reduction in cardiac arrests and
duration of clinical instability after implementation of a paediatric
rapid response system. Quality & Safety in Health Care. 2009;18:500-4.
11. Hunt EA, Zimmer KP, Rinke ML, Shilkofski NA,
Matlin C, Garger C, et al. Transition from a traditional code
team to a medical emergency team and categorization of cardiopulmonary
arrests in a childrens center. Arch Pediatr Adolesc Med.
2008;162:117-22.
12. Brilli RJ, Gibson R, Luria JW, Wheeler TA, Shaw
J, Linam M, et al. Implementation of a medical emergency team in
a large pediatric teaching hospital prevents respiratory and
cardiopulmonary arrests outside the intensive care unit. Pediatric
Critical Care Medicine. 2007; 8:236-46.
13. Sharek PJ, Parast LM, Leong K, Coombs J, Earnest
K, Sullivan J, et al. Effect of a rapid response team on
hospital-wide mortality and code rates outside the ICU in a Childrens
Hospital. JAMA. 2007;298:2267-74.
14. Tibballs J, Kinney S, Duke T, Oakley E, Hennessy
M. Reduction of paediatric in-patient cardiac arrest and death with a
medical emergency team: preliminary results. Arch Dis Child.
2005;90:1148-52.
15. Lobos AT, Costello J, Gilleland J, Gaiteiro R,
Kotsakis A, Ontario Pediatric Critical Care Response Team Collaborative.
An implementation strategy for a multi-center pediatric rapid response
system in Ontario. Joint Commission Journal on Quality & Patient Safety.
2010; 36:271-80.
16. Critical Care Secretariat, Ontario MHLTC. CCRT
user utilization survey. 2011.
17. Franklin C, Mathew J. Developing strategies to
prevent inhospital cardiac arrest: analyzing responses of physicians and
nurses in the hours before the event. Crit Care Med. 1994 ;22:244-47.
18. Rich K. Inhospital cardiac arrest: pre-event
variables and nursing response. Clinical Nurse Specialist.
1999;13:154-6.
19. Coombs M, Dillon A. Crossing boundaries,
re-defining care: the role of the critical care outreach team. J Clin
Nurs. 2002;11:387-93.
20. Cioffi J. Recognition of patients who require
emergency assistance: A descriptive study. Heart Lung J Acute Crit Care.
2000;29:262-68.
21. Schmid A, Hoffman L, Happ MB, Wolf GA, DeVita M.
Failure to rescue: a literature review. J Nurs Adm. 2007;37:188-98.
22. Neal R, Mohamed Ali A, Gaiteiro R, Hussain S, Parshuram C,
Kotsakis A, et al. Does a designated paediatric critical care
response team reduce readmission rates to paediatric critical care?
Pediatr Crit Care Med. 201;12(3 Suppl. 1):A18.

