cute and persistent diarrhea have
been associated with increased intestinal permeability, and
repeated episodes of diarrhea result in malnutrition in
children [1-3]. To study the association between diarrhea,
malnutrition and intestinal function we evaluated intestinal
permeability in children admitted with acute gastroenteritis
and controls with no gastrointestinal illness.
All children aged 6-59 months,
hospitalized for management of acute gastroenteritis,
without co-morbid conditions and with a weight >5 kg, were
eligible for recruitment as cases. For each case, a child
admitted at the same time for an illness other than severe
infection or gastrointestinal disease was recruited. Written
informed consent was obtained from parents and the study was
approved by the institutional review board. Clinical
management was according to regular protocols, with oral or
intravenous rehydration as indicated. All children had
anthropometric measurements recorded using calibrated
weighing scales and infantometer and underwent the
lactulose-mannitol test for intestinal permeability.
Malnutrition was defined as a weight for
age Z score below -2SD by WHO Anthro [4]. For the
lactulose-mannitol test, after a 3-hour fast, children were
given 2 mL/kg body weight of test solution containing
50mg/ml of mannitol and 250 mg/mL of lactulose. The entire
volume of urine passed in the 5 hour period following the
test solution was collected using adhesive urine bags in a
container with 1 mL of chlorhexidine. The volume was
measured and a 10 mL aliquot stored in a sterile 15 mL
polypropylene tube at -20°C until testing. The ratio
of urinary excretion of lactulose to mannitol was measured
by high performance liquid chromatography (Ultra Fast Liquid
Chromatography System, Shimadzu, Spinco Biotech, Chennai)
with evaporative light scatter detection using melibiose as
an internal standard. The values of lactulose mannitol in
urine are expressed as the ratio % excretion of lactulose /
% excretion of mannitol (or LM ratio) during the 5 hours,
and a ratio of greater than or equal to 0.089 indicates
increased permeability [5,6]. Fischer’s exact test was used
to compare proportions between groups
 |
|
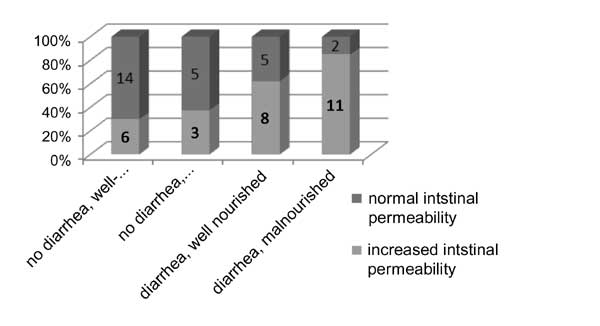
Fig. 1. Distribution of
children in each category.
|
A total of 64 children were enrolled,
with 34 cases and 30 controls. Ten children were excluded,
five because of inadequate sample collection and five
because of problems with determining the LM ratio. Thus,
data from 54 children were analysed, with 26 cases and 28
controls. There were differences in age, weight and height
in children enrolled as cases and controls, with controls
being older (mean age of 28 months vs. 19 months),
and consequently taller (mean height 84.7 cm vs. 79.1
cm) and heavier (10.7 kg vs. 8.8 kg), but there were no
differences in the gender distribution (67% male vs.
73% male) or nutritional status (mean WAZ score -1.5 vs.
-1.8). The LM test showed increased permeability in 61.5% of
children with acute diarrhea and 32.2% of children without
diarrhea (P=0.01). When the children were stratified
by nutritional status, 78.6% of children with malnutrition
and acute diarrhea and 37.5% of malnourished controls had
increased intestinal permeability.
Mannitol is absorbed transcellularly and
lactulose has a paracellular route of absorption. Reduction
in mannitol absorption shows reduced surface area and
increased lactulose absorption indicates a leaky gut [7]. In
this study, use of the LM test in a south Indian population
showed that a third of all children have abnormal intestinal
permeability, there was an expected increase of permeability
in children with acute diarrhea and alteration in intestinal
permeability was greater in children with concurrent
malnutrition and diarrhea. We acknowledge the limitations of
small numbers and differences between cases and controls.
Malnutrition and diarrhea form a vicious
cycle leading to worsening of the malnutrition state and
outcome of diarrheal infections [8], which may potentially
be prevented with better nutrition or mechanisms such as by
tightening cell junctions or promoting rapid repair of
intestinal epithelium.
Acknowledgments: This study was
undertaken by RTB for the Short Term Studentship 2010
program under the Indian Council of Medical Research. I
would like to thank Dr Anup Ramachandran for his critical
input in preparation of the paper, Ms Sophia J for
conducting the LM assay, Sr Charlet and Sr Margeret for data
and sample collection and clinical care and Drs Rajiv
Sarkar, Rahul Thomas and Azara Singh for their critiques,
guidance and support.
Contributors: RTB: Compiled
data, prepared the letter; AJJ: Lent critical guidance; Kang
G: Planned the study and lent critical help and AB: Planned
and supervised the study.
Funding: Fluid Research
Grant-Research Committee, Christian Medical College,
Vellore; Competing interests: None stated.
References
1. Welsh FK, Farmery SM, MacLennan K,
Sheridan MB, Barclay GR, Guillou PJ, et al. Gut
barrier function in malnourished patients. Gut.
1998;42:396-401.
2. van Elburg RM, Uil JJ, de Monchy JG,
Heymans HS. Intestinal permeability in pediatric
gastroenterology. Scand J Gastroenterol Suppl.
1992;194:19-24.
3. Barboza Junior MS, Silva TM, Guerrant
RL, Lima AA. Measurement of intestinal permeability using
mannitol and lactulose in children with diarrheal diseases.
Braz J Med Biol Res. 1999;32:1499-1504.
4. WHO Multi-Centre Child Growth
Reference Study Group. WHO Child Growth Standards based on
length/height, weight and age. Acta Paediatr Suppl.
2006;450:76–85.
5. Bao Y, Silva TMJ, Guerrant RL, Lima
AM, Fox JW. Direct analysis of mannitol, lactulose and
glucose in urine samples by high-performance anion-exchange
chromatography with pulse amperometric detection. Clinical
evaluation of intestinal permeability in human
immunodeficiency virus infection. J Chromatogr B Biomed
Appl. 1996; 685: 105-12.
6. Lima AA, Silva TM, Gifoni AM, Barrett
LJ, McAuliffe IT, Bao Y, Fox JW, Fedorko DP, Guerrant RL.
Mucosal injury and disruption of intestinal barrier function
in HIV-infected individuals with and without diarrhea and
cryptosporidiosis in northeast Brazil. Am J Gastroenterol.
1997;92:1861-6.
7. Hollander D. Intestinal permeability,
leaky gut and intestinal disorders. Curr Gastroenterol Rep.
1999;1:410-6.
8. Petri WA Jr, Miller M, Binder HJ, Levine MM,
Dillingham R, Guerrant RL. Enteric infections, diarrhea, and
their impact on function and development. J Clin Invest.
2008;118:1277-90.

