|
|
|
Indian Pediatr 2011;48: 19-24 |
 |
Short Course Versus 7-Day Course of
Intravenous Antibiotics for Probable Neonatal Septicemia:
A Pilot, Open-label, Randomized Controlled Trial |
|
Shiv Sajan Saini, Sourabh Dutta, Pallab Ray* and Anil Narang
From the Division of Neonatology, Department of Pediatric
and *Department of Medical Microbiology, Postgraduate Institute of Medical
Education and Research, Chandigarh, India.
Correspondence to: Dr Sourabh Dutta, Additional
Professor, Department of Pediatrics, PGIMER,
Chandigarh 160012, India.
Email: [email protected]
Received: September 9, 2009;
Initial review: October 9, 2009;
Accepted: January 28, 2009.
Published online: 2010 August 1.
PII: S097475590900636-1
|
|
Abstract
Objective: To compare a short course of
antibiotics (48 to 96 hours) and a standard course of antibiotics (7
days) for probable neonatal sepsis.
Design: Randomized, controlled, open-labeled
trial with blocking and stratification according to birth weight.
Setting: Tertiary care, referral, teaching
hospital in Northern India.
Participants: Neonates >30 wks gestation and
>1000 g at birth, with probable sepsis (clinical signs of sepsis, raised
C-reactive protein) were enrolled. Babies with major malformations,
severe birth asphyxia, meningitis, bone or joint or deep-seated
infection, those who were already on antibiotics, and those undergoing
surgery were excluded. Neonates, who had clinically remitted on
antibiotic therapy – by the time a sterile blood culture report was
received – were randomized.
Intervention: In the intervention arm,
antibiotics were stopped after the 48-hour culture was reported sterile.
In the control arm, antibiotics were continued to a total of 7 days.
Main outcome measure: "Treatment failure"
defined as reappearance of signs suggestive of sepsis within 15 days of
stopping antibiotics, supported by laboratory evidence and adjudicated
by a blinded expert committee.
Results: 52 neonates were randomized to
receive a short course or 7-day course (n=26 each). Baseline
variables were balanced in the 2 groups. There was no significant
difference in the treatment failures between the 2 groups (3 babies in
the 7-day group vs none in short course group, P=0.23).
Conclusion: No difference in the treatment
failure rates could be identified between short course and 7-day groups
among neonates >30 weeks and >1000 grams with probable sepsis.
Key words: Antibiotics, Duration, Neonatal sepsis, Short
course, Treatment failure.
|
|
D
uration of appropriate antibiotic
therapy for neonatal sepsis does not have evidence-based guidelines.
Standard textbooks recommend treatment end points of 7-14 days for blood
culture positive or clinically probable infections [1-3]. The rationale
and safety of these recommendations have never been scientifically
evaluated.
Such untested approaches could result in the
unnecessary use of antibiotics leading to increased cost of care,
unnecessary intravenous catheterization, prolonged hospitalization,
mother-infant separation, increased colonization by pathogenic organisms
and emergence of drug-resistant strains [4-7]. A shorter duration of
antibiotic therapy may benefit by decreasing the above undesirable
consequences [8]. We hypothesized that 7 days of antibiotics might be too
long in cases of probable neonatal sepsis. The protection offered by
longer duration of antibiotics may be offset by the above mentioned
drawbacks.
Considering the ambiguity in the existing literature
about guidelines for the duration of antibiotic therapy for probable (i.e.
non-culture-proven) neonatal sepsis, we conducted this trial to determine
whether a policy of stopping antibiotics early incurs a significantly
higher treatment failure rate than conventional seven days therapy.
Methods
This was a controlled, open-label, randomized trial
with stratification and blocking, conducted between September 2006 to
November 2007 at a Level III neonatal unit in Northern India. The unit has
a large referral load and caters to a middle-to-low socioeconomic
population. The study was approved by the Institute’s Ethics Committee.
All inborn or outborn neonates, admitted in the
Neonatal Unit with birth weight >1000 grams and gestation age >30 weeks,
who were started antibiotics for probable sepsis were eligible for study.
Both early onset and late onset sepsis were included. The diagnosis of
probable sepsis was based on the presence of a repertoire of clinical
symptoms and signs over duration of at least 6 hours and a positive
C-reactive protein (CRP) test. The clinical signs were recorded by the
chief investigator. Positive CRP was defined as presence of agglutination
at a dilution of ³1:2
by a semi-quantitative latex agglutination test (Teco Diagnostics, 1268, N
Lake view Ave, Anaheim, CA. 92807 USA), which corresponded to a CRP titre
of ³12
mg/L. Babies with major congenital malformations, severe birth asphyxia
(defined as Apgar score
£3
at 5 minutes), meningitis [9], clinically suspected bone/joint/deep seated
localized infection, those who were already on antibiotics for a previous
episode of sepsis, and those undergoing surgery were excluded from the
study.
An information sheet providing the details of the study
was provided to the parents. Identification, demographic and clinical
details of the sepsis episode and CRP results were recorded in a
structured case report form after taking written informed consent.
Sample size: Ideally, this research question
merits a non-inferiority trial where sample sizes are huge. Baseline
treatment failure rate after 7 days of antibiotics for probable sepsis is
not reported in literature. Hence we planned to have a sample size of 50
patients as a pilot study. To account for 10% loss during follow up, we
decided to recruit 55 patients.
Randomization was done between 48 and 96 hours after
the enrollment, if the following randomization criteria were fulfilled:
Clinical signs of sepsis had remitted; Blood culture was reported sterile
after 48 hours or more of incubation. (An upper limit of 96 hours for
reporting was kept to account for the occasional delay in reporting over
weekends or non-office hours); and CSF analysis was not suggestive of
meningitis [9].
Stratification was done for birth weight (1000-1500 g
and >1500 g). Each stratum consisted of permuted blocks of randomly
varying sizes. Eligible babies were randomly allocated in a 1:1 ratio to
one of the two groups: Short-course group: these subjects did not
receive further antibiotics after receiving the blood culture report, or
7-day group: these subjects received a total of 7 days of
antibiotics. The random allocation sequence was computer generated and
slips of paper bearing the allocated intervention were placed in serially
numbered, opaque, sealed envelopes to ensure concealment of allocation.
One of the investigators generated the allocation sequence and another
enrolled and assigned participants. As consecutive patients got enrolled,
the opaque envelope was opened and the intervention was executed.
Routine and supportive care was provided in a similar
fashion to patients in both groups as per unit guidelines. Antibiotics
were prescribed as per the policy prevalent in the unit at that time. The
use of breast milk is aggressively promoted in our unit. Intra-venous
fluids are stopped once milk intake crosses 100-120 mL/kg/day.
We monitored the subjects for episodes of sepsis in
follow-up. The period of observation was 15 days after completion of
antibiotics. All subjects were followed up by weekly appointments. At each
visit, information regarding episodes of illnesses in the previous week
was recorded by the chief investigator. If any subject did not come for
follow-up, they were contacted by telephone. Parents were asked to report
to our unit for any episode of illness till 15 days. The clinical signs
and symptoms were noted by chief investigator and a detailed structured
proforma was filled for all such episodes. A sepsis screen, blood culture,
chest X-ray, CSF and other relevant work up were done for all such
episodes. A two-member, blinded, adjudication committee of experienced
neonatologists reviewed these forms and masked chest radiographs. Each
member gave his/her opinion independently whether the episode of illness
represented bacterial septicemia. In cases where they held divergent
opinions, a consensus was arrived upon by mutual consultation.
Outcome variables: The key outcome variable was
"treatment failure" occurring within 15 days of stopping antibiotics and
was defined as reappear-ance of signs suggestive of sepsis, supported by
laboratory evidence and adjudicated to be relapse by a blinded expert
committee.
Statistical analysis: The baseline variables were
described by descriptive statistics. As all outcome variables were
categorical, c2
test with Yates correction or Fisher’s Exact Test, as applicable, were
used. P value <0.05 was taken as significant. We analyzed subjects
as per intention to treat. Analysis was done using SPSS version 13.0 and
Microsoft Excel 2003.
Results
Out of 305 neonates who fulfilled the inclusion
criteria (Fig. 1), only 65 met exclusion criteria and 188
could not meet randomization criteria. Hence, 52 babies were randomly
allocated to the short-course and the 7-day antibiotic group (26 babies
each). Ten babies got enrolled in the 1000-1500 grams and 42 in the >1500
grams strata. Baseline variables were comparable between the two study
groups (Table I). No baby received TPN during study period.
All cases, randomized to either group, completed their respective courses
of appropriate antibiotics with full compliance. Over the 15 days
follow-up period, there was 1 loss to follow-up in each group.
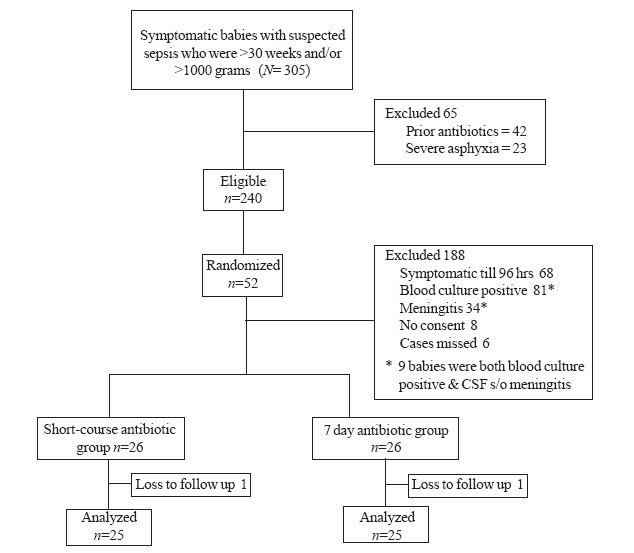 |
|
Fig. 1 Study flow.
|
TABLE I
Comparison of Baseline Variables
|
Baseline variable |
Short-course group |
7-day group |
| |
(n=26) |
(n=26) |
|
Gestational age (wks), Mean ± SD |
34.9 ± 3.3 |
34.6 ± 3.4 |
|
Birthweight (g), Median (IQR) |
1900(1627, 2478) |
1687(1504, 2450) |
| Age at
onset of symptoms (hrs), Median (IQR) |
19 (0, 96) |
47 (1, 342) |
|
Enrollment (d), Median (IQR) |
3 (1, 4) |
3 (2, 15) |
|
Randomization (d), Median (IQR) |
5.5 (4, 8) |
6 (4, 18.25) |
| Duration
of symptoms (hr), Mean ± SD |
48.0 ± 24.4 |
58.7 ± 24.1 |
| EOS:LOS |
17:9 |
17:9 |
| 1 Min
Apgar, Median (IQR) |
8 (6, 8) |
8 (7, 8) |
| 5 Min
Apgar, Median (IQR) |
9 (8, 9) |
9 (8, 9) |
| Rupture
of membranes ³24 hours |
3 (11.5%) |
3 (11.5%) |
| Maternal
fever |
3 (11.5%) |
0 |
| Mothers
received antibiotics before delivery |
7 (27%) |
3 (11.5%) |
EOS: early-onset sepsis; LOS: late onset sepsis; IQR: Interquartile range.
|
The two groups were balanced in terms of the signs and
symptoms of sepsis at presentation. The use of antibiotics was similar
between the two groups. Group 2 neonates received Amikacin more often than
group 1 (P=0.03). A greater number of babies in the short-course
group received supplemental oxygen, whereas more babies in the 7-day group
received continuous positive airway pressure (CPAP). All other supportive
interventions were used in similar proportions in the two groups. (Table
II).
TABLE II
Comparison of Co-interventions
|
Co-intervention |
Short-course group |
7-days group |
P |
| |
(n=26)(%) |
(n=26)(%) |
value |
| Any
cephalosporin |
24(92.3) |
24(92.3) |
1.00 |
| Amikacin |
18(69.23) |
24(92.3) |
0.03 |
|
Cloxacillin |
6(23.07) |
1(3.8) |
0.10 |
|
Supplemental oxygen |
14(53.85) |
5(19.23) |
0.02 |
| CPAP |
1(3.8) |
8(30.8) |
0.02 |
| NIMV |
1(3.8) |
1(3.8) |
1.00 |
| Any
respiratory support |
16(61.5) |
12(46.15) |
0.26 |
| DVET |
1(3.8) |
1(3.8) |
1.00 |
| Plasma
products |
1(3.8) |
1(3.8) |
1.00 |
| Dextrose
infusion |
1(3.8) |
0 |
1.00 |
Figures in parentheses are percentages; CPAP- continuous positive airway pressure,
NIMV- Nasal intermittent mandatory ventilation, DVET- Double volume exchange transfusion.
|
Three babies in the 7-day group had treatment failure
as opposed to none in the short-course group (P=0.23). One baby
developed apnea 10 days after stopping antibiotics. His blood culture grew
methicillin resistant Staphylococcus aureus. Another baby
got admitted with complaints of lethargy, poor feeding and diarrhea 10
days after stopping antibiotic therapy and had evidence of meningitis (50
white blood cells per µL, all
neutrophils). Both babies recovered on antibiotic therapy. The third baby
was discharged on day 13 of life (4 days after stopping antibiotics) and
was asymptomatic at discharge. The baby died unexpectedly at home a couple
of days later.
Discussion
In this study, there was no significant difference in
the treatment failure rates with short course and 7 days of antibiotics
for uncomplicated probable neonatal sepsis. Hence it generates the
possibility of shortening of duration of antibiotic therapy in probable
neonatal sepsis.
We included only symptomatic babies and did not include
asymptomatic babies with maternal risk factors as they are likely to have
a very low baseline event rate and would show a favorable response
irrespective of duration of antibiotics. This is in contrast to previous
serial CRP based studies which included babies irrespective of
symptomatology [10-13]. We excluded extremely low birth weight babies as
they often have subtle signs of sepsis which can be clinically missed and
various other neonatal diseases may mimic sepsis, making the evaluation of
treatment failure a difficult exercise. We used CRP (at presentation) as a
marker of sepsis and did not use serial CRP values to stop antibiotics, so
that the results could be generalized to even resource-poor areas, where
laboratory facilities are not easily available.
In the present study, randomization was done only if
the babies had become completely asymptomatic. It would be impossible to
exclude persisting infection with reasonable certainty in symptomatic
babies and, thus, would be unethical to stop antibiotics. We did not
randomize at the beginning of the antibiotic course because there was no
way of predicting which babies would become asymptomatic by the time the
culture report was available. This study replicated the state of clinical
dilemma (regarding continuation of antibiotics) that exists after a baby
who had suspected sepsis with raised CRP, becomes asymptomatic soon after
starting antibiotics.
An upper limit of 96 hours was decided based on the
observations made in previous studies [10,14]. To minimize measurement
bias, the diagnosis of "treatment failure" in our study was adjudged by
two blinded neonatologists. Since the study entailed a new regimen of a
potentially fatal disease, we took special measures to ensure that the
treatment failures were not missed.
The non-significant trend of higher treatment failures
in the 7-day group was, if anything, reassuring, that in this limited
sample the short course regime was not worse than the 7-day regime. The
possible reason of the increased treatment failures could be presence of
IV cannula for a longer duration in 7 days group, when it was not
required. Although the two groups were balanced in terms of baseline
variables, neonates in 7-day group appear to be born lighter and sicker
(higher proportion were lethargic, had abdominal distension and poor
feeding), which could also partly contribute to the outcome. A sampling
error may have occurred due to small sample size. The study has certain
limitations. The sample size of our study was too small for us to conclude
that a short course of antibiotics is definitely not inferior to a
standard 7-day course. Using our study as a pilot study, we estimate the
sample size for a non-inferiority trial to be approximately 2700. The
results are only valid for the specified subgroup of preterm neonates of
>1000 g birth weight and >30 weeks gestation. We could not introduce
blinding as it was impractical to prepare identical-looking placebos for a
wide range of antibiotics, and arrange for sham antibiotic administration
for the short course group. Quantitative CRP assay would have been more
useful as a marker for sepsis.
In the current study, there was no statistically
significant difference in the treatment failure rates between a short
course and a 7-day course of antibiotics among preterm neonates >30 weeks
and >1000 grams with probable sepsis, who became asymptomatic within 48 to
96 hours of intravenous antibiotics.
Contributors: SSS: Collected data, entered
data, did analysis, wrote first draft; SD: Conceived the idea, planned the
study, finalized the manuscript; PR: Performed blood cultures; AN:
Supervised study, edited the manuscript.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Duration of antibiotic therapy for neonatal
sepsis has no evidence base, but 7 to 10 days of antibiotics are
often prescribed for probable sepsis.
What This Study Adds?
• Within the limitations of a small sample size,
the treatment failure rate with a short course of antibiotics
(between 48-96 hours) was not worse than that with a 7-day course of
antibiotics among neonates with probable sepsis, who become rapidly
asymptomatic with antibiotic therapy.
|
References
1. Schelonka RL, Freij BJ, McCrecken GH Jr. Bacterial
and fungal infections. In: MacDonald MG, Seshia MMK, Mullett MD,
editors. Avery’s Neonatology. Pathophysiology and management of the
newborn. 6th ed. Philadelphia: Lippincott William Wilkins. 1999. p.
1235-75.
2. Klein JO. Bacterial sepsis and meningitis. In:
Remington JS, Klein JO, editors. Infectious Diseases of Fetus and Newborn
Infant. 5th Ed. Philadelphia: WB Saunders & Co. 2001.p.943-99.
3. Dear P. Neonatal infections: Infections in the
newborn. In: Rinnie JM editor. Roberton’s Textbook of Neonatology.
4th ed. Elsevier Churchill Livingstone; 2005. p. 1011-1093.
4. Hammerschlag MR, Klein JO, Herschel M, Chen FC,
Fermin R. Patterns of use of antibiotics in two newborn nurseries. N Engl
J Med. 1977;296:1268-9.
5. Philip AG, Hewitt JR. Early diagnosis of neonatal
sepsis. Pediatrics. 1980;65:1036-41.
6. Goldmann DA, Leclair J, Macone A. Bacterial
colonization of neonates admitted to an intensive care environment. J
Pediatr. 1978;93:288-93.
7. Lacey RW. Evolution of microorganisms and antibiotic
resistance. Lancet. 1984;2:1022-5.
8. Low DE, Scheld WM. Strategies for stemming the tide
of antimicrobial resistance. JAMA. 1998;279:394-5.
9. Gray JE, Ringer SA. Common neonatal procedures.
Cloherty JP, Eichenwald EC, Stark AR editors. In: Manual of
Neonatal Care. 5th ed. Philadelphia: Lippincott William Wilkins.
2004.p.687-702.
10. Ehl S, Gering B, Bartmann P, Hagel J, Pohlandt F.
C-reactive protein is a useful marker for guiding duration of antibiotic
therapy in suspected neonatal bacterial infection. Pediatrics.
1997;99:216-21.
11. Bomela HN, Ballot DE, Cory BJ, Cooper PA. Use of
C-reactive protein to guide duration of empiric antibiotic therapy in
suspected early neonatal sepsis. Pediatr Infect Dis J. 2000;19:531-535.
12. Khashabi J, Karamiyar M, Taghinejhad H, Shirazi M.
Use of serial C-reactive protein measurements for determination of the
length of empiric antibiotic therapy in suspected neonatal sepsis. Iran J
Med Sci. 2004;29: 31-5.
13. Lee WE, Chan ML, Young BWY. Reducing
hospitalization and antibiotic use in suspected early neonatal sepsis
through serial measurements of C-reactive proteins. HK J Pediatr.
2005;10:3-9.
14. Engle WD, Jackson GL, Sendalbach D, Ford D, Oleson
B, Burton KM, et al. Neonatal pneumonia: comparison of 4 Vs 7 days
antibiotic therapy in term and near-term infants. J Perinatol.
2000;20:421-6.
|
|
|
 |
|

