Megalencephalic leukoencephalopathy with subcortical
cysts (MLC), also known as van der Knaap’s disease, is characterized by
early-onset macrocephaly, with mild motor developmental delay and
seizures; gradual onset of ataxia, spasticity, and sometimes
extrapyramidal findings; and usually late onset of mild mental
deterioration.
Macrocephaly is present at birth or develops during
the first year of life. The degree of macrocephaly is variable and can
be as much as 4-6 SD above the mean. Almost all patients have epilepsy
from an early age. Some patients have died in their teens or twenties
but others are alive in their forties.
Case Reports
Case 1: A 4-year-old boy, born of second degree
consanguineous marriage in a Muslim community, with uneventful birth
history, presented with progressively increasing head size noticed from
1 year of age, and left sided simple focal seizures from 2 years of age.
He attained social smile by 4 months and head control by 6 months of
age. He could walk with support and was able to speak a few words by 2½
years, which is his current status too. He had not attained bladder or
bowel control.
On examination, he had a head circumference of 54.5
cm (>95th percentile). He was able to comprehend, obey commands and
could speak a few words only. He had bipyramidal signs. Sensory system
was normal and there were no cerebellar signs. His optic fundi were
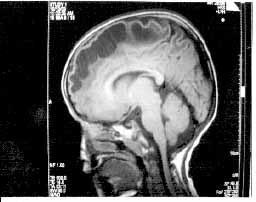
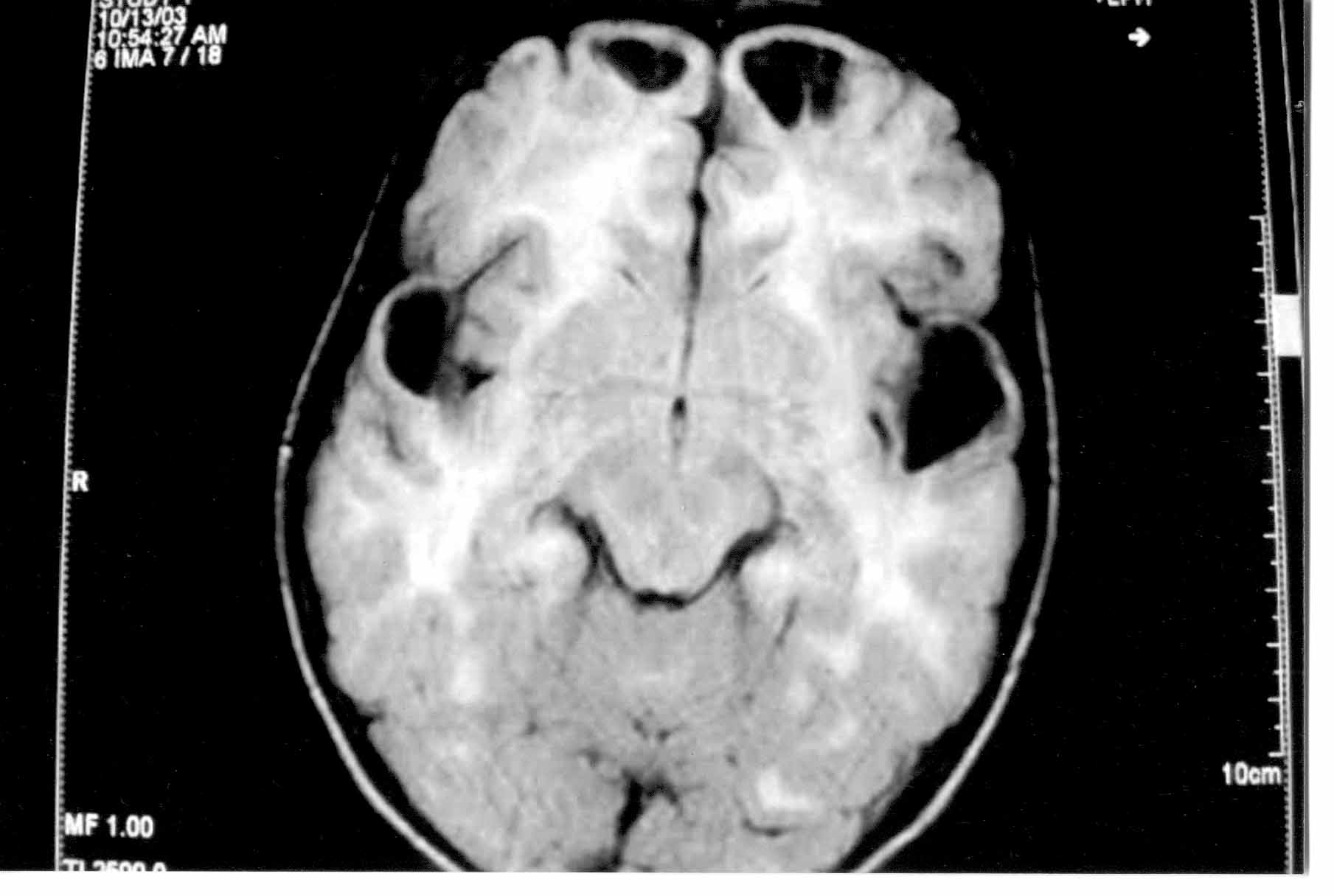
normal, there was no cherry-red spot and did not have organomegaly. MRI
brain (Figs.1 & 2) revealed bilaterally symmetrical white matter
changes with extensive sub-cortical cysts in frontal, anterior temporal
and parietal regions, consistent with a diagnosis of MLC.
 |
 |
|
Fig. 1. MRI brain sagittal view showing extensive subcortical
cystic changes and sparing of central white matter structures. |
Fig. 2. MRI brain showing bilaterally symmetrical white matter
changes and cysts in frontal and anterior temporal region. |
Case 2: A 2-year-old younger sibling of the
patient mentioned above had similar complaints. She had increasing head
circumference from 1 year of age and generalized seizures since 1 year
of age. She had motor and mental developmental delay. She had a head
circumference of 52 cm (>95th percentile) and spasticity in both lower
limbs. Her MRI brain revealed identical findings.
Urine metabolic screening was done, for both the
patients, which was negative. EEG showed bilateral generalized
epileptiform activity in both cases. No other family members were
affected with any such neurological illness even in the 3 past
generations. Both the patients were treated symptomatically with use of
anti-convulsants and physiotherapy.
Discussion
Megalencephalic leukoencephalopathy with subcortical
cysts was first described by van der Knaap, et al. in 1995(1).
MLC is a rare disease with a low carrier rate. The disease has a high
incidence in populations in which consanguinity is common(2-4).
MLC is an autosomal recessive disorder due to
mutations in MLCI gene(5,6) which has its locus in chr22qter. The
physiological function of the protein is at present unknown. It is
probably an integral membrane protein.
The diagnosis of MLC can be made with confidence in
patients with typical clinical findings and characteristic abnormalities
on cranial MRI. Macrocephaly is present at birth or, more commonly,
develops within the first year of life in all patients. Early
development is normal or mildly delayed. Most children achieve
independence in walking. Slow deterioration of motor functions with
cerebellar ataxia and mild spasticity usually starts in early
childhood. The majority of the patients become wheelchair dependent in
their teens. Some patients have extrapyramidal movement abnormalities
with dystonia and athetosis, usually as a late finding. Mental decline
occurs later and is much milder than motor decline. Most patients have
epileptic seizures.
The MRI is diagnostic. MRI of the brain shows
diffusely abnormal, mildly swollen cerebral hemispheric white matter.
Central white matter structures, including the corpus callosum, internal
capsule, and brain stem are better preserved, although they are not
usually entirely normal. Subcortical cysts are invariably present in the
anterior-temporal region and often in the frontoparietal region. Over
time, the white matter swelling decreases and cerebral atrophy ensues.
The subcortical cysts may increase in size and number.
The differential diagnosis of MLC includes Canavan’s
disease, Alexander disease, infantile-onset GM2 and GM1 gangliosidosis
and merosin-deficient con-genital muscular dystrophy.
In Canavan’s disease, MRI shows involvement of the
thalamus and globus pallidus, which are spared in MLC(7). The white
matter may be cystic, but the typical subcortical cysts are lacking.
Alexander disease leads to a megalencephaly and leuko-encephalopathy
with frontal predominance of MRI abnormalities and contrast enhancement
of particular brain structures, which is not a feature of MLC(8). Cystic
degeneration may occur in Alexander’s disease, but deep frontal white
matter is mainly affected. MLC characteristically has an early onset and
slow progression, whereas Canavan’s and Alexander’s disease have a rapid
progression. MRI in infantile GM2 gangliosidosis is characterized by
prominent involvement of the basal ganglia and thalami in addition to
the white matter abnormalities. MRI features in infantile GM1
ganglio-sidosis(9) are very similar to those of GM2 gangliosidosis.
Demonstration of deficiency of beta-galactosidase activity in leukocytes
confirms the diagnosis.
So far, all attempts to treat MLC have failed.
Patients have been treated with acetazolamide, but neither the clinical
symptoms nor the white matter swelling improved. Supportive therapy
includes the prescription of anticonvulsants if the patient has
seizures. Physical therapy is important to improve motor dysfunction.
Special education is required for many patients.
Prenatal diagnosis is possible by analysis of DNA
extracted from fetal cells obtained by amniocentesis at 16-18 weeks’
gestation or chronic villus sampling at about 10-12 weeks’ gestation.
Contributors: HK and LP were involved in
conception and design, acquisition of data, analysis and interpretation
of data and in drafting of the manuscript. GK and TKVM were involved in
critical revision of the manuscript for important intellectual content.
GK gave final approval of the version to be published and will act as
guarantor for the article.
Funding: None.
Competing interests: None declared.
