From the Division of Pediatric Nephrology,
Department of Pediatrics, All India Institute of Medical Sciences, New
Delhi 110 02, India.
Correspondence to: Dr. Arvind Bagga, Department of
Pediatrics, All India Institute of Medical Sciences, Ansari Nagar, New
Delhi 110029, India. E-mail:
[email protected]
In order to examine the etiology of refractory
rickets, we evaluated the case records of patients presenting
between 1990 and 2002. Subjects with impaired renal functions were
excluded. Of 131 patients, 25.9% each had hypophosphatemic rickets
and distal renal tubular acidosis (RTA), 19.6% vitamin D dependent
rickets (VDDR), 11.3% proximal RTA, 9.1% liver disease and 6.1%
malabsorption. A significant proportion of patients with VDDR and
proximal RTA showed deformities in the first year of life, whereas
those with distal RTA and hypophosphatemic rickets presented
later. Patients with hypophosphatemic rickets had predominant
involvement of lower limbs; hypercalciuria was found in 4. Distal
RTA was associated with marked rickets and normal levels of
alkaline phosphatase. Hypophosphatemia and low tubular
reabsorption of phosphate, though characteristic of
hypophosphatemic rickets, was also seen in patients with VDDR
(19.2%) and distal RTA (17.6%). Our findings suggest that
application and interpretation of appropriate investigations are
useful in determining the cause of non-azotemic refractory rickets
allowing initiation of specific therapy.
Key words: Hypophosphatemic rickets, Renal tubular
acidosis, Vitamin D dependent rickets.
Nutritional deficiency of vitamin D and calcium
is the most common cause of rickets in children and responds
satisfactorily to its treatment. Rickets secondary to
hypophos-phatemia, vitamin D dependence, renal tubular acidosis (RTA),
liver disease, malabsorption and chronic renal failure does not
respond to therapy with vitamin D, and is defined as refractory
rickets(2). An accurate diagnosis of the underlying cause of
refractory rickets allows initiation of specific therapy, often with
satisfactory results.
There is paucity of published data on the
etiology of refractory rickets in children. We report our experience
on the etiology of this condition in patients attending this
hospital during the last 13 years. While children with chronic renal
failure comprise a significant proportion of subjects with
refractory rickets, this diagnosis is readily established following
estimation of blood levels of serum creatinine. Differentiation
between other conditions is more difficult and was the subject of
the study.
Subjects and Methods
Case records of children referred for evaluation
of refractory rickets to the Renal Metabolic Clinic of the All India
Institute of Medical Sciences during 1990 to 2002 were reviewed.
Rickets was defined radiologically as presence of widened and
irregular epiphyseal-metaphyseal junctions or evidence of bone
softening (bowing) in long bones(3) and elevated levels of serum
alkaline phosphatase. Since patients with distal RTA and bony
deformities often show normal blood levels of alkaline phosphatase,
the presence of clinical and radiological features of rickets was
considered sufficient for the diagnosis(4).
Subjects with rickets were treated with a total
dose of 6,00,000 IU of vitamin D administered orally over a period
of 10 days. An X-ray wrist was done, three weeks later, for
healing of rickets, which was defined by the radiological presence
of the line of healing. Patients not showing healing were retreated
with same dose followed by an X-ray 3 weeks later. Patients
who failed to show radiological healing despite two doses of vitamin
D were diagnosed as refractory rickets(2). Patients with impaired
renal functions [creatinine clearance < 50 mL/min/1.73 m2,
calculated by Schwartz formula(5)], deranged liver functions (blood
bilirubin level >2 mg/dL and/or aspartate or alanine trans-aminase
>5 times normal) or malabsorption (history of diarrhea, and abnormal
D-xylose test and/or high 24 hr fecal fat) were excluded.
The case notes were reviewed for age at onset of
symptoms and family history of similar disease. The height, weight,
pattern of skeletal deformities, dentition and findings on systemic
examination were noted. Eye examination for cataract, cystine
crystals, KF ring and intraocular tension was performed in all
patients. Blood levels of calcium, phosphate, alkaline phosphatase,
urea, creatinine, albumin, pH, bicarbonate, electrolytes, bilirubin
and transaminases were estimated. Urinalysis and measurement of
calcium, phosphate, creatinine, sugar and albumin was done on timed
and spot specimens; urine was also examined for abnormal
aminoaciduria. Tubular maximum of phosphate factored by GFR (TmPO4/GFR)
was determined on timed urine collection using normograms(6).
Hypercalciuria was diagnosed when the 24-hour urinary calcium
excretion exceeded 4 mg/kg. Patients with metabolic acidosis (pH <
7.25 or base excess > 5 mEq/L) were further evaluated with urine
pH. Fractional excretion of bicarbonate and urine to blood CO2
difference were determined after bicarbonate loading in these
subjects(7). Intact parathormone (PTH) levels were measured in
subjects with normal blood levels of calcium, creatinine, pH and
bicarbonate. Diagnostic criteria for etiology of refractory rickets
are shown in Table I.
TABLE I
Criteria for Etiology of Refractory Rickets.
Condition
|
Criteria
|
Vitamin D dependent rickets
|
Normal or low serum phosphate*, serum calcium < 8.5 mg/dl
and/or high intact blood PTH (> 150 pg/ml)
|
Hypophosphatemic rickets
|
Low serum phosphate*, low TmPO4/GFR!, normal blood
calcium and PTH levels
|
Distal RTA(7)
|
Metabolic acidosis (pH < 7.25, base excess > 5 mEq/L),
urine pH > 5.5
On bicarbonate loading (at blood bicarbonate > 22 mEq/L):
fractional excretion of bicarbonate <10%
urinary to blood PCO2 difference <10 mm Hg
|
Proximal RTA(7)
|
Metabolic acidosis (pH < 7.25, base excess > -5 mEq/L),
urine pH < 5.5
On bicarbonate loading (at blood bicarbonate >22 mEq/L):
fractional excretion of bicarbonate >15%
urinary to blood PCO2 difference >10 mm Hg
|
| Fanconi
syndrome |
Proximal
RTA with glucosuria, aminoaciduria and low
TmPO4/GFR |
* Normal range for blood phosphate levels(7) 0-5 days of life: 4.8-8.2 mg/dL,
6 days-4 years: 4-6.8 mg/dl, 4-11 yr: 3.7-5.6 mg/dL, 12-15 yr: 2.9-3.4 mg/dL.
TmPO4/GFR(6) 0-1 month: 4-10.7 mg/dL, 1-3 months: 4-9.5 mg/dL,
3-6 months: 4-8.2 mg/dL, 6 months-5 yr: 2.9-4.6 mg/dL, 5-12 year: 2.8- 4.4 mg/dL.
Data are represented as mean (95% confidence
interval, CI) unless otherwise stated. Significance of difference in
means was tested using one-way analysis of variance (ANOVA).
Results
Of 260 records reviewed, complete clinical and
biochemical workup was available in 241. One hundred and ten
patients with impaired renal functions (n = 110) were excluded from
the study. The underlying diagnosis in remaining patients was hypo-phosphatemic
rickets in 34 patients (25.9%), vitamin D dependent rickets (VDDR)
in 26 (19.8%), distal RTA in 34 (25.9%), proximal RTA in 15 (11.5%),
liver disease in 12 (9.2%) and malabsorption in 8 (6.1%). Pseudo-hypoparathyroidism
was diagnosed in 2 siblings with hypocalcemia, hyperphospha-temia,
normal blood levels of creatinine and elevated parathormone. Data of
patients with hypophosphatemic rickets, vitamin D dependent rickets
and RTA is presented.
TABLE II
Clinical Features in Chief Etiological Categories.
| |
Distal RTA
n = 34
|
Proximal RTA
n = 15 |
Vitamin D dependent
rickets n = 26
|
Hypophosphatemic
rickets n = 34
|
Boys : Girls
|
19 : 15
|
13 : 2
|
11 : 15
|
15 : 19
|
Age at onset (yr)*
|
3 (2-4)
[1 mo-10 yr]
|
2 (0.7-3.3)
[1 mo-10 yr]
|
1.9 (1.1-2.7)
[18 days-9 yr]
|
2.7 (2.1-3.3)
[1-10 yr]
|
Onset <1 yr
|
12
|
7
|
13
|
3
|
Onset >1 yr
|
22
|
8
|
13
|
31
|
|
Clinical features |
Polyuria
|
34
|
12
|
|
|
Fractures
|
7
|
|
3
|
3
|
Enamel hypoplasia
|
3
|
|
7
|
3
|
Seizures
|
|
|
8
|
|
Families affected
|
6
|
2
|
1
|
4
|
1. Tetany was seen in 6 subjects and alopecia in 2 with vitamin D dependent rickets.
2. Hypokalemic muscle weakness was seen in 3 patients with distal RTA.
* Expressed as mean (95% confidence interval) [range].
Clinical features (Table II)
The mean age at onset of skeletal deformities was
similar in all etiological groups. Of 8 patients who presented
before the age of 3 months, 5 had VDDR while 3 had proximal RTA.
Most patients with hypo-phosphatemic rickets (31 out of 34, 91.2%)
and distal RTA (22 out of 34, 64.7%) presented after the age of 1
year. Rickets was incidentally diagnosed during evaluation for
seizures (7 with VDDR), polyuria and polydipsia (4 with distal RTA),
hypotonia (2 with proximal RTA due to Lowe syndrome) and
extrapyramidal involvement (1 with proximal RTA secondary to Wilson
disease).
The pattern of skeletal deformities was similar
in children with VDDR and RTA with involvement of both upper and
lower limbs. A significant number of patients with hypophosphatemic
rickets (19 out of 34) showed clinical involvement of lower limbs
with sparing of the upper limbs and skull.
Proximal RTA was isolated in 2 (13.3%) and
associated with Fanconi syndrome in 13 (86.7%). Fanconi syndrome was
secondary to Lowe syndrome in 2 and Wilson disease in 1. Distal RTA
was considered primary in all cases; none of the patients had
deafness. Hypophosphatemic rickets was secondary to McCune Albright
syndrome with fibrous dysplasia in one subject.
Biochemical Investigations in Refractory Rickets.
Parameter
|
Distal RTA
(n = 34)
|
Proximal RTA
(n = 15)
|
Vitamin D dependent
rickets (n = 26) |
Hypophosphatemic
rickets (n = 34)
|
Calcium mg/dL*
|
9.4 (9.1 + 9.7)
|
9.5 (9-10)
|
6.9 (6.4-7.4)
|
9.6 (9.4-9.8)
|
|
[7.9-11.8]
|
[8.1-10.7]
|
[4.2-8.5]
|
[8.6-11.3]
|
Phosphate mg/dl #
|
3.2 (3-3.4)
|
2.7 (2.3-3.1)
|
3.5 (3.2-3.8)
|
2.5 (2.3-2.7)
|
|
[1.9-4.4]
|
[1.6-4.3]
|
1.5-4.8
|
[1.4-3.4]
|
Alkaline phosphatase
|
866 (611-1121)
|
1196 (944-1448)
|
1749 (1356-1842)
|
1209 (955-1463)
|
IU/L @
|
[199-4212]
|
[342-2222]
|
[655-4530]
|
[207-4645]
|
Hypophosphatemia
|
6
|
10
|
5
|
34
|
Hypocalcemia
|
3
|
4
|
26
|
|
Hypercalciuria
|
24
|
8
|
|
4
|
Nephrocalcinosis
|
11
|
|
|
1
|
Aminoaciduria
|
4
|
12
|
2
|
|
Values represent mean (95% CI) [range].
* Significantly lower value for vitamin D dependent rickets (VDDR) compared to other
categories (P <0.001).
# Significantly lower values in hypophosphatemic rickets and proximal RTA compared to
VDDR and distal RTA (P <0.001).
@ Significantly lower values compared to VDDR in distal RTA (P = 0.003) and
hypophosphatemic rickets (P = 0.04).
Laboratory profile (Table III)
Serum calcium levels were lower in patients with
VDDR compared to other conditions. Low serum calcium levels (<8.5
mg/dL) were also present in 4 (26.7%) patients with proximal RTA and
3 (8.8%) with distal RTA. Low serum phosphate and reduced TmPO4/GFR
were found in all patients with hypophosphatemic rickets, 5 (19.2%)
with VDDR and 6 (17.6%) with distal RTA. Serum alkaline phosphatase
levels were lower in children with distal RTA compared to other
patients, with 22 (64.4%) having levels in the normal range (<840 IU/L).
Radiological evidence of fractures was present in 7 (20.6%) patients
with distal RTA, 3 (11.5%) with VDDR and 3 (8.8%) with
hypophosphatemic rickets.
Metabolic acidosis was more severe in distal RTA
{mean blood pH - 7.21 (95% CI 7.19 - 7.23) [range 7.04 - 7.3]} than
proximal RTA {mean blood pH 7.26 (95% CI 7.23-7.29) [range
7.1-7.34]} (P = 0.03). The mean blood bicarbonate levels were 13.9
(95% CI 13 - 14.8) [range 9.5 - 19 mEq/L] and 15.8 (95% CI 14.6 -
17) [range 12 - 19 mEq/L] respectively (P = 0.002). Mean fractional
excretion of bicarbonate in subjects with distal RTA was 5.5% (95%
CI 5.1 - 5.9%) [range 4 - 8%] compared to 23% in proximal RTA (95%
CI 20.6 - 25.4) [range 15.5-33%] (P = 0.001). Ultrasound abdomen
showed features of medullary nephrocalcinosis in 11 (32.4%) patients
with distal RTA and one (2.9%) with hypophosphatemic rickets.
Hypercalciuria was seen in 24 (70.6%) patients with distal RTA, 8
(53.3%) with proximal RTA and 4 (11.8%) with hypophosphatemic
rickets(3).
Discussion
The chief causes of refractory rickets in the
present study included chronic renal failure, hypophosphatemic
rickets, distal and proximal RTA, vitamin D dependent rickets, and
liver disease. These findings show that etiology of refractory
rickets can be deter-mined with proper application and
interpretation of available laboratory findings. Specific therapy is
possible in most instances making establishment of diagnosis a
desirable goal.
Hypophosphatemic rickets was an important cause
of refractory rickets in the present study. Presentation after
infancy with predominant involvement of lower limbs and absence of
hypocalcemia is characteristic, as was also observed in our
patients(9). Treatment of these patients comprises of administration
of phosphate supplements and vitamin D analogs. Clinicians should,
however, be aware of the subgroup of patients with hypercalciuria,
as seen in 4 subjects in this report(10). Therapy of the latter is
restricted to phosphate supplements; treatment with vitamin D
analogs is avoided in view of the risk of nephrocalcinosis.
Bone disease in distal RTA has classically been
described as mild osteopenia, while frank rickets is not common(3).
The presence of severe bony deformities, including pathological
fractures, in patients in the present study, is therefore
significant. Despite presence of frank radiological changes of
rickets, almost two-third patients showed normal blood levels of
alkaline phosphatase. Blood levels of alkaline phosphatase are index
of bone formation. Since persistent metabolic acidosis, in patients
with distal RTA, results in reduced rate of bone formation, blood
levels of the enzyme may be normal.
The etiology of rickets in distal RTA is not
clear. The rachitic deformities resolve follow-ing alkali
supplementation and treatment with vitamin D is not necessary(11).
While chronic metabolic acidosis is speculated to adversely affect
vitamin D metabolism, blood levels of vitamin D metabolites are
reported to be normal(12). Caldas, et al. in a study on 28
patients with distal RTA proposed that the vitamin D status might
determine the severity and extent of bony deformities(13). Low
dietary intake of calcium and phosphorus is common in school-going
children in our country(14), and if associated with reduced vitamin
D stores may contribute to rickets and an increased the risk of
fractures in these patients.
VDDR characterized by impaired synthesis (type I)
or resistance to the action of 1, 25 dihydroxyvitamin D3 (type
II)(15), was also an important cause of refractory rickets in our
subjects. While estimation of blood levels of vitamin D metabolites
was not possible in our patients, the presence of hypocalcemia and
elevated blood levels of PTH were sufficient to differentiate VDDR
from other causes, including hypophosphatemic rickets. Response to
treatment with potent vitamin D analogs (alpha-hydroxyvitamin D or
1, 25 dihydroxyvitamin D) is useful for differentiating between VDDR
types I and II(16).
Hypocalcemia characteristically seen in all
patients with VDDR was also found in 26.7% and 8.8% patients with
proximal and distal RTA respectively. Impaired metabolism of vitamin
D and hypercalciuria may variably contribute to hypocalcemia in
subjects with RTA. Similarly, while hypophosphatemia and low TmPO4/GFR
is characteristic of phos-phate losing states (hypophosphatemic
rickets and proximal RTA), this was also present in 17.6% and 19.2%
of our patients with distal RTA and VDDR respectively. These
biochemical abnormalities are attributed to a phosphaturic effect of
secondary hyper-parathyroidism in the latter conditions, rather than
primary phosphate wasting.
Our findings emphasize that hypo-phosphatemia and
increased urinary excretion of phosphate is not diagnostic of hypo-phosphatemic
rickets. Detailed clinical assessment and appropriate investigations
(blood pH, bicarbonate, calcium and PTH) are necessary for
establishing the correct diagnosis.
A limitation of this report concerns our not
having screened all subjects for biochemical evidence of fluorosis.
Fluorosis is endemic in this part of the country, attributed chiefly
to high fluoride levels in ground water(17). Skeletal fluorosis may
affect school going children, resulting in bony deformities and
radiological changes that mimic rickets; osteosclerosis is uncommon.
Based on the early age of onset of symptoms in this study, the
possibility of skeletal fluorosis however seems remote. While most
patients with skeletal fluorosis present in late childhood or
adolescence(18), the mean age patients in the present study was 2.5
yr [90.1% below 5 years].
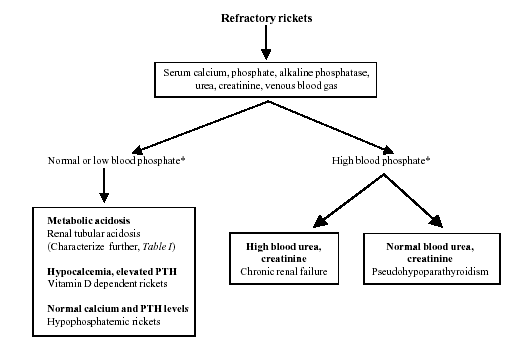
Our study emphasizes the need for a standard
protocol at every referral center for evaluating patients with
refractory rickets. The protocol followed in this hospital is shown
in Fig 1. Initial investigations include estimation of blood
levels of calcium, phosphate, alkaline phosphatase, creatinine, pH,
bicarbonate and total CO2. The presence of metabolic acidosis on 2
or more occasions suggests the diagnosis of RTA, which should be
characterized further. Blood levels of calcium and PTH levels are
useful in differentiating between VDDR and hypo-phosphatemic
rickets.
 |
|
Fig. 1. Evaluation of
refractory rickets. |
* Related to age specific norms (see Table I)
Contributors: MM, PH and AB were involved in
management of patients. AB, AnB and AdB planned the study. AnB and
AdB collected data. AnB performed literature review and drafted the
manuscript. AB critically reviewed the manuscript and will act as
its guarantor.
Funding: None.
Competing interest: None.
