Surendra
Kumar
Sunita J. Ferns*
B. Vishnu Bhat*
D.K. Patro**
From
the Departments of Pathology, Pediatrics* and Orthopedics**,
JIPMER, Pondicherry 605 006, India.
Correspondence to: Dr. Surendra
Kumar, Associate Professor, Department of Pathology, JIPMER,
Pondicherry 605 006, India. Email: [email protected]
Manuscript Received:
January 21, 2002;
Initial review completed: May 22, 2002;
Revision Accepted: September 27, 2002.
A 10-year-old
male child presented with multiple lymph node swellings. A
diagnosis of Hodgkins disease was made on histopathological
examination. The patient developed relapse six months after his
last chemotherapy as a solitary bone tumer, which is rare.
Immunohistochemical evaluation helped for the correct typing of
Hodgkins disease.
Key words:
Bone tumor, Hodgkins disease.
A 10-year-old male
child presented with multiple lymph node swellings. A diagnosis of
Hodgkins disease was made on histopathological examination. The
patient developed relapse six months after his last chemotherapy
as a solitary bone tumer, which is rare. Immunohistochemical
evaluation helped for the correct typing of Hodgkins disease.
Key words: Bone
tumor, Hodgkins disease.
Hodgkins disease
(HD) rarely presents as a solitary bone tumor(1). Although HD can
spread to the bones in late stages causing destructive lesions,
presentation with a solitary bone lesion is uncommon(2). Fewer
than 20 such cases have been reported in literature and most of
them were reported prior to the development of immunological
markers for Hodgkins disease and T and B cell lymphoma.
Immunological markers are necessary for accurate typing of the
disease. We report a child previously diagnosed as Hodgkins
disease and successfully treated, who later presented with
solitary bone tumor in the upper part of right humerus.
Case Report
A 10 year old male
child presented with multiple lymph node swellings in the axillary
and cervical regions. He was diagnosed as a case of Hodgkins
disease (mixed cellularity type) based on histopathological
examination of the cervical lymph node. His ultrasound abdomen and
whole body radiographs taken at the time of initial diagnosis were
unremarkable. He was staged Ann Arber II and was put on CHOP
regime consisting of cyclophosphamide 750 mg/m2 (D1), adriamycin
50 mg/m2(D1), oncovin 1.4 mg/m2(D1) and prednisolone
100mg/m2(D1-5). Six such cycles were adminstered at a gap of 21
days. Though initially he appeared to respond very well to this,
he returned six months after his last chemotherapy cycle with
complaints of progressive swelling over the right shoulder for 3
months. Physical examination at this presentation revealed a 5 ×
4 × 3 cm size hard swelling over the right shoulder fixed to the
humerus. The skin over the swelling was normal. There were no
enlarged lymph nodes or hepato-splenomegaly. The radiograph showed
lytic lesions over the right upper humerus. A clinical diagnosis
of osteosarcoma of humerus was made. The patient’s hematological
profile revealed a hemoglobin of 8.5 g/dL, a total count of
10,500/cu mm and a differential count of P40L35E15M7B3. The
peripheral smear showed normocytic, normochromic red blood cells
and adequate platelets. The patient was subjected to fine needle
aspiration cytology.
On microscopic
examination the smears revealed good cellularity. There were
classical binuclear and mononuclear Reed-Sternberg cells and
plasma cells in lymphoid background. A few eosinophils,
neutrophils and histiocytes were also seen. A diagnosis of
Hodgkins disease was made and confirmed by histopathological
examination subsequently. Immunohistochemical analysis revealed
staining of the atypical cells for CD15 and CD30. The cells were
negative for S-100, Keratin, CD-45, CD3, CD4 and CD4sRO. The tumor
was excised and he was put on a BACOP regime i.e. bleomycin-5
mg/m2iv(D15, 22), adriamycin-25 mg/m2iv(D1, 8),
cyclophosphamide-650 mg/m2iv(D1,8). vincristine 1.4 mg/m2iv(D1,8)
and prednisolone 60 mg/m2(D15-22). Six such cycles were given at
an interval of 28 days each and the site involved was irradiated
along with the axilla with 1500 rads of radiation using a 4 MeV
linear accelerator. The child is doing well after one year of
follow up.
Discussion
Approximately 3% of
primary malignant bone tumors are lymphomas. Non Hodgkins lymphoma
of large B cell type are the most common type of lymphomas(4).
Hodgkins disease can spread to the bones in terminal stages. Bone
marrow involvement is common in Hodgkins disease, but this does
not usually produce bone tumors. The lesions in Hodgkins disease
have been described in detail by several authors(2,5).
Radiographic evidence of bone involvement is seen in about 14% of
Hodgkins disease. Lytic, sclerotic or mixed bone lesions may be
present in Hodgkins disease. Sometimes periosteal new bone
formation is also seen(6). Multifocal bone involvement, stage IV
is usually due to hematogenous dissemination(6). But sometimes
direct invasion from an adjacent lymph node leads to a small
solitary lesion (Stage 1E). The prognosis in stage 1E is similar
to that of local nodal disease without osseous involvement.
Newcomer et al.(7)
observed 24% lytic, 20% sclerotic and 15% mixed lesions in their
study. The remaining 41% were unclassifiable. Granger et al(2)
demonstrated 75% lytic lesions and only 5% mixed periosteal
reactions. Sclerotic lesions are thought to indicate hematogenous
dissemination, whereas lytic lesions may be the result of direct
extension from adjacent lymph nodes(8). The most commonly involved
bones are the ileum followed by vertebrae, ribs, and proximal
femur(1). In the present case proximal humerus was involved which
is unusual. The term primary Hodgkins disease of bone is commonly
used to describe those cases presenting with osseous
involvement(3,9). Many cases reported in literature had clinically
detectable local or distant lymph node involvement, suggesting
that true primary Hodgkins disease of the bone is quite rare.
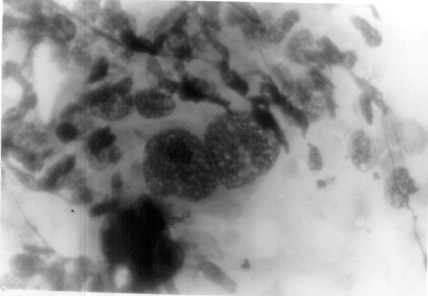 |
| Fig.1. Photomicrograph
showing classical binucleate Read sternberg cell in lymphoid
background (MGGx400). |
Solitary bone
lesion at the time of diagnosis is an uncommon presentation in
Hodgkins disease. In our case, the boy presented with cervical and
axillary lymphadenopathy and other constitutional signs and
symptoms of Hodgkins disease initially, whereas later on, in
relapse only solitary bone lesion was found at the right upper end
of humerus. We had not come across any such case in literature who
presented with solitary bone lesion during relapse, although there
are a few case reports of Hodgkins disease initially presenting as
solitary bone tumor.
The patient was put
on chemotherapy as well as radiotherapy because patients with
lymphoma of the bone especially with a previous systemic disease
are better treated as for systemic disease. In a study(10) done at
the Dana Farber Center on 11 children treated with radiation and
chemotherapy consisting of APO (adriamycin, prednisolone and
oncovin), the 8 year actuarial lymphoma free survival was 90%.
There were no relapses. In the bone tumor center Bologna, Italy 23
out of 26 patients (88%) were disease free following radiation and
chemotherapy (adriamycin, vincristine and cyclophosphamide) with
no local relapses at 7.5 year median follow up(11). The survival
of patients in Hodgkins disease of the bone receiving a combined
modality treatment is around 84%. However we have found no
statistics for the survival rate for patients with relapse in bone
presenting as tumor.
In our case the
immunohistochemical pattern was typical of Hodgkins disease
(nodular sclerosis type). At the time of initial diagnosis
immunocytochemistry studies were not done and the diagnosis of
mixed cellularity was based on morphological picture alone. It is
possible that the intial tumor was also a nodular sclerosis in its
early stages, however in the absence of an initial
immunocytochemistry report we can not be sure. We are of the
opinion that Hodgkins disease should be considered in the
differential diagnosis of solitary bone lesions particularly if
radiological findings are indicative. Immunohistochemical studies
are useful in the accurate typing.
Acknowledgement
The authors are
grateful to Dr. Naresh of Tata Memorial Hospital, Mumbai for
conducting the immunohistochemical examination of the specimen.
Contributors:
SK, SF and BVB drafted the manuscript. SK conducted the
pathological examination. DKP conducted the surgery and managed
the patient.
Funding:
None.
Competing interests:
None stated.
1. Ozdermirli M,
Mankin HJ, Aisenberg AC. Harris NL. Hodgkin’s disease
presenting as a solitary bone tumor. A report of four cases and
review of the literature. Cancer 1996; 77: 79-88.
2. Granger W,
Whitaker R. Hodgkin’s disease in bone, with special reference
to periosteal reaction. Br J Radiol 1967; 40: 939-948.
3. Burke JS.
Hodgkins disease: histopathology and differential diagnosis. In:
Neoplastic hematopathology, 1st ed. Ed Knowles DM. Baltimore,
Williams and Wilkins, 1992; pp 497-533.
4. Ostrowski ML,
Unni KK, Banks PM, Shives TC, Evans RG, O’Connell MJ, et al.
Malignant lymphoma of bone. Cancer 1986; 58: 2646-2655.
5. Horan FT. Bone
involvement in Hodgkins disease. A survey of 201 cases. Br J
Surg 1969; 56: 277-281.
6. Kaplan HS.
Hodgkins disease. 2nd edition. Cambridge, Harvard University
Press. 1980; pp 220-222.
7. Newcomer LN,
Silverstein MB, Cadman EC, Farber LR, Bertino JR, Prosnitz LR.
Bone involvement in Hodgkin’s disease. Cancer 1982; 49:
338-342.
8. Parker BR,
Marglin S, Castellino RA. Skeletal manifestations of leukemia,
Hodgkins disease, and non Hodgkins lymphoma. Semin Roentgenol
1980; 15: 302-315.
9. Kadin ME.
Hodgkins disease: immunobiology and pathogenesis. In:
Neoplastic Hematopathology, 1st ed. Eds Knowles DM. Baltimore,
Williams and Wilkins, 1992; pp 535-554.
10. Loeffler JS,
Tarbell NJ, Kozakewich H, Cassady JR, Weinstein HJ. Primary
lymphoma of bone in children: analysis and treatment results
with adriamycin, prednisolone, oncovin (APO), and local
radiation therapy. J Clin Oncol 1986; 4: 496-501.
11. Bacci G, Jaffe N, Emiliani E,
Van Horn J, Manfrini M, Picci P, et al. Therapy for
primary non Hodgkin’s lymphoma of bone and a comparison of
results with Ewing’s sarcoma. Ten years experience at the
Istituto Ortopedico Rizzoli. Cancer 1986; 57: 1468-1472.
|
