|
|
|
Indian Pediatr 2015;52:
135-140 |
 |
Small for Gestational Age: Growth and Puberty
Issues
|
|
Sangita Yadav and D Rustogi
From Department of Pediatrics, Maulana Azad Medical College and
Associated Lok Nayak Hospital,New Delhi, India.
Correspondence to: Dr Sangeeta Yadav, Director Professor, Department
of Pediatrics, Maulana Azad Medical College (MAMC), Bahadur Shah Zafar
Marg, New Delhi 110 002, India.
Email:
[email protected]
|
Context: Small for gestational age infants have
multifold increased risk of growth failure and adulthood disorders.
Those who experience rapid catch up growth are at risk of developing
metabolic syndrome, whereas those without catch up may end up with short
stature. These children are also prone to an altered pubertal
development.
Need and Purpose: Scarcity of literature, lack of
published guidelines on the follow-up and management plan of children
born with small for gestational age.
Evidence Acquisition: Literature search in PubMed
was conducted with regard to epidemiology, growth and puberty,
comorbidities, its pathogenesis and management in small for gestational
age, with particular relevance for developing countries. An algorithm
for follow-up of these children is outlined, based on available empiric
data.
Conclusions: Being born small for gestational age
predisposes to many metabolic and pubertal disorders. Special emphasis
is needed for early detection and management through early surveillance
in growth clinics, and regular follow-up to prevent associated
comorbidities.
Keywords: Intrauterine Growth retardation, Puberty, Short
stature.
|
|
B
eing born small for gestational age (SGA), either
according to weight or length, is a risk factor for growth and
development disorders, and chronic diseases later in life. The term’
small for gestational age’ represents a statistical group of infants
whose weight and/or crown-heel length is less than expected for their
gestational age and sex [1]. The definition of SGA is not
straightforward as it requires accurate knowledge of gestational age,
measurements of birth
weight, length, and head circumference, and a
reference data from a relevant population. The
cut-off has been variably set at the 10th centile, 3rd centile,
or at less than –2 SD from the mean (approximately
2nd centile) [2]. The International Small for Gestational Age Advisory
Board Consensus Development Conference Statement 2001, and the Consensus
Statement of the International Societies of Pediatric Endocrinology and
the Growth Hormone Research Society 2007, recommend that SGA should be
defined as a neonate whose birth weight or birth crown-heel length is at
least 2 standard deviations (-2 SD) below the mean for the gestational
age, based on data derived from a reference population [2,3]. Although
segregation of SGA from normal is somewhat arbitrary, -2 SD was selected
because it encompasses the majority of patients with disordered fetal
growth. These babies can be sub- classified into SGA for weight,
length, or for both weight and length [3]. SGA
must be differentiated from low birth weight and intrauterine growth
retardation (IUGR). A newborn short for his/her gestation may or may not
be low birth weight. The term SGA refers not to fetal growth but to the
size of the infant at birth whereas, the term IUGR suggests diminished
growth velocity in the fetus occurring in utero, as documented by
at least two intrauterine growth assessments. A child who is born SGA
has not necessarily suffered from IUGR, and infants who are born after a
period of IUGR are not necessarily SGA [4]. Separating small babies, who
are small simply as a result of adaptation to maternal size, from those
who have suffered IUGR, presents a diagnostic challenge. It is the IUGR
group that is at an increased perinatal risk
[5]. Pitfalls in recording accurate period of
gestation, birth length and also non-availability of these records for
later review are major challenges.
SGA children are at higher risk of attaining an adult
height below their target height, as well as of developing metabolic
disorders – obesity, diabetes and cardiovascular diseases [6,7]. These
children are also prone to have precocious pubarche, exaggerated
precocious adrenarche, an earlier onset of menarche, and faster
progression of puberty than children born appropriate for gestational
age (AGA) [8]. Developmental sequelae affecting the GH-IGF axis and
adrenal and gonadal function are seen in children with abnormal weight
gain during infancy and childhood
[6,7]. Tempo of this postnatal weight gain is emerging as
particularly important in the relationship between birth weight and
adult diseases [6]. There is lack of data not only on the SGA-associated
co-morbidities, but also associated awareness about regular, long term
follow up of these children in special clinics.
Epidemiology
The data on the incidence of SGA births are scarce in
many countries because birth length and gestational age are rarely
recorded in National databases. Based on the available data, it has been
estimated that between 2.3 and 10% of all infants are born SGA [9].
India has a high incidence of low birth weight (LBW) and SGA
babies [10-12]. The incidence of LBW in India is about 30% babies in
contrast to 5-7% in developed countries [10]. A large percentage
(approximately 70%) of LBW are SGA [10,13]. Kushwaha, et al.
[11] studied 750 hospital deliveries (term
singleton neonates) and found that 28.4% were SGA, which is almost
similar to the incidence of 25% reported by Mehta, et al. [12].
Thus there is a huge burden of LBW and SGA in our country which needs to
be addressed.
The causation of SGA is multifactorial. Fetal factors
include chromosome abnormalities and genetic defects. Maternal factors
involve age, weight and height, parity, chronic diseases, infections,
impairment of nutritional status, and substance abuse. Placental factors
include structural abnormalities and insufficient perfusion. Thus the
ability to reach an optimal birth weight results from the interaction
between the fetal growth potential (the fetal factors) and the
environment (placental and maternal factors) [2]. The definition of SGA
does not take into account the background growth-modifying
factors such as maternal size, ethnicity, and
parity. These factors may help in understanding
the mechanisms and implications of being born SGA.
Narang, et al. [14] in 1997 concluded that
idiopathic intrauterine growth retardation is the commonest cause of SGA
in Indian babies, followed by pregnancy induced hypertension which is
one of the most important risk factors for SGA/IUGR.
Growth in SGA
About 90% of SGA children show some degree of
accelerated growth during infancy. In this context, rapid infant growth
can be viewed as a compensatory mechanism for prenatal growth deficit,
referred to as ‘Catch-up growth’. Catch up growth is defined as weight
or length gain greater than 0.67 SD score, which represents the width of
each percentile band in standard growth charts, indicating clinically
significant centile crossing [15,16]. Catch-up is typically an early
postnatal process that in most SGA infants is completed by the age of
two years. Different growth patterns may be identified in infants as
young as three months [6]. While 80% of infants born SGA show catch-up
growth during the first 6 months of life, 90% have catch-up growth with
a height SD score of more than –2 by two years of age. Approximately 10%
do not show catch-up growth, and most of these children continue to
experience poor growth throughout childhood and remain short after the
age of two years [4,17]. These individuals constitute a relatively high
proportion of children and adults with short stature with a relative
risk of 5-7 times than children born at normal size [17,18]. Karlberg,
et al. [4] reported seven-fold increased risk of growth failure
in SGA children, and it is said to contribute to 20% of the short adult
population.
The mechanisms that allow catch-up growth in SGA
children or prevent them from achieving normal height are still largely
unknown. Nutritional or environmental insults in perinatal life can
cause irreversible, long-term outcomes. The timing of such insults is
significant in determining the extent of later adverse consequences to
health. Three peptide hormones that share structural homology (IGF-I and
-II and insulin) seem to be the most important endocrine regulators in
early postnatal life. Low, et al. [19] suggested that catch-up
growth in SGA children might be, at least in part, affected by
intrauterine reprogramming of hypothalamic-pituitary-adrenal axis.
Mother’s height and weight are an important determinant of the adult
height and weight of their children. SGA birth and their subsequent
growth may also be the result of poor maternal nutrition, which is
common in developing countries.
There is paucity of data on growth patterns in Indian
SGA infants. There has been only one long term study on follow-up of low
birth weight (LBW) infants reported from India, which was started in the
late 60’s on hospital- born
urban cohort [10]. On evaluation of 79 premature AGA and 45 full-term
SGA children, they found that the SGA remained significantly affected in
their overall physical growth even at 14 years. In an unpublished study
conducted at our institute over a period of two years from 2010-2012, of
the 110 SGA babies enrolled between 12-18 months with mean age of 15
months, 62.7% (69) babies showed catch-up growth either in weight,
length or both, and 37.3% (41) did not show any catch-up. On further
stratification, 21.8% (24) showed catch up only in weight, 10.9% (12)
only in length, and 30% (33) showed catch-up both in weight and length.
Thus a total of 51.8% babies showed catch-up in weight and 40.9% in
length [20,21].
Puberty in SGA
Puberty is one of the most important milestones in
life, and involves growth spurt, changes in body shape and physiological
functions. Being born SGA predisposes to a number of pubertal disorders
like precocious adrenarche and puberties, and earlier onset of menarche.
The timing as well as progression of puberty is linked to being born
SGA. The main differences between the pubertal growth patterns of SGA
and AGA children are that accelerated bone maturation and peak height
velocity occur at an earlier pubertal stage in SGA children, resulting
in a shorter duration of pubertal growth and a smaller than expected
pubertal growth spurt. Though bone age maturation starts earlier in SGA
children, it is not a reliable predictor of height potential in these
children [8]. The important determinants of final height are the height
and age at onset of puberty and the magnitude and duration of the
pubertal growth [22], but the studies are scarce. Low birth weight is a
risk factor for the later development of abdominal or truncal obesity,
and SGA children with catch-up weight gain show a dramatic transition
toward central adiposity, which enhances insulin resistance [23]. The
sequence from low birth weight to precocious pubarche has been proposed
to be a classic referral point in the progression to an early menarche
followed by a polycystic ovary syndrome phenotype and, ultimately a
shorter adult height [8]. One of the possible mechanisms responsible for
this sequence may be early accumulation of visceral fat following
postnatal catch-up growth, leading to insulin resistance and
hyperinsulinism, which is thought to play a pivotal role in the
development of a hyperandrogenic state in SGA girls [24]. Adiponectin,
IGFBP-1 and triglycerides have also been implicated in the
pathophysiology of obesity-related insulin resistance, glucose
intolerance, and insulin-mediated lipoprotein metabolism.
Hypoadiponectinemia has been associated with linear catch-up and is
involved in pathogenesis of insulin resistance in SGA children; this may
lead to precocious pubarche but only limited and conflicting information
is available [23]. Jaquet, et al. [24] have also highlighted the
critical contribution of adipose tissue in the metabolic complications
in the SGA patient, with long-term consequences.
Children who show rapid postnatal weight gain have
the highest adrenal androgen levels. In a retrospective Australian study
of 89 children with precocious pubarche, 35% of the children were born
SGA. The authors concluded that being born SGA according to weight
and/or length is an independent risk factor for precocious pubarche
[25]. Among the possible causes underlying this association are
increased central adiposity, decreased insulin sensitivity and increased
IGF-I levels between the ages of 2 and 4 years in SGA children with
excess weight gain. According to the Avon Longitudinal Study of Parents
and Children (ALSPAC), the combination of low birth weight and rapid
postnatal weight gain had predicted increased total and central
adiposity and higher IGF-I levels at 5 years of age, and lower insulin
sensitivity at 8 years of age [26].
Most authors agree that puberty in short SGA children
starts at a normal age, but relatively early for their short stature
[27], yet the results are difficult to compare due to variations in SGA
definitions, inclusion criteria, methodologies and follow-up periods.
Several longitudinal follow-up studies comparing different groups of SGA
and AGA children did not find any significant difference in the
progression of puberty or age at menarche between girls born SGA and AGA
[18,28]. However, other studies showed an earlier age of menarche in
girls with fetal growth restriction relative to girls born with
appropriate birth weight [29]. Ibanez, et al. [30] found that
menarche before the age of 12 yrs was 3-fold more prevalent among girls
born SGA (n=50); their age at menarche was advanced by 8-10
months compared with girls of normal birth weight. In an Indian study by
Bhargava, et al. [10], menarche occurred 6 months earlier in the
preterm group and 12 months earlier in the SGA group than in full-term
AGA controls. Persson, et al. [31] reported that boys and girls
born SGA were on an average 4 cm shorter at the onset of puberty than
children without perinatal risk factors. Thus there is some evidence
that pubertal height gain may be lesser than expected in children born
SGA.
Consequences of Being Born SGA
Among SGA children who do not achieve catch-up growth
by 2 year of age, the relative risk of short stature at 18 year of age
is 5.2 for those born light and 7.1 for those born short [17].
Low birth weight due to fetal growth retardation, and
SGA children who experience rapid catch-up growth during childhood have
been linked to development of the metabolic syndrome with all its
diverse components (referred to as insulin resistance syndrome) – type 2
diabetes, hypertension, obesity, and hyperlipidemia. Barker, et al.
[32] observed that the risk of metabolic syndrome at the age of 50 yr
was 10-fold greater in individuals with a birth weight less than 2.5 kg
than in those whose birth weight exceeded 4.5 kg. In another study,
there were statistically significant differences in all components of
the metabolic syndrome at 22 yr of age between the SGA and the AGA
groups [33]. They found that 2.3% of individuals born SGA develop
metabolic syndrome according to Adult Treatment Panel III criteria,
compared with only 0.3% of individuals born AGA. Furthermore, insulin
resistance was significantly associated with other indicators of the
metabolic syndrome, such as a high waist-to-hip ratio, hypertension,
hypertriglyceridemia, and hyperglycemia [33]. Pubertal comorbidities in
SGA are; higher risk for polycystic ovary syndrome, fertility problems,
ovarian dysfunction, reduced fertility and early menopause [34,35].
Follow-up Plan of SGA Babies
SGA babies should have a regular follow-up in high
risk clinic for monitoring of their weight and length to prevent
consequences of short stature, metabolic syndrome and altered puberty.
The algorithm for follow-up and early surveillance has been briefly
outlined in Fig. 1. Regular monitoring of weight, height,
body mass index, and pubertal assessment during adolescence is
particularly important. It is imperative to prevent excessive weight
gain, which can be achieved through exclusive breast-feeding till 6
months, adequate maternal nutrition both intra- as well as post-partum
and consumption of a low fat balanced diet as per individual’s energy
requirements. Breast feeding till two years of age not only slows the
rate of weight gain in infancy, but also has a protective effect on
long-term risk of obesity and intellectual impairment.
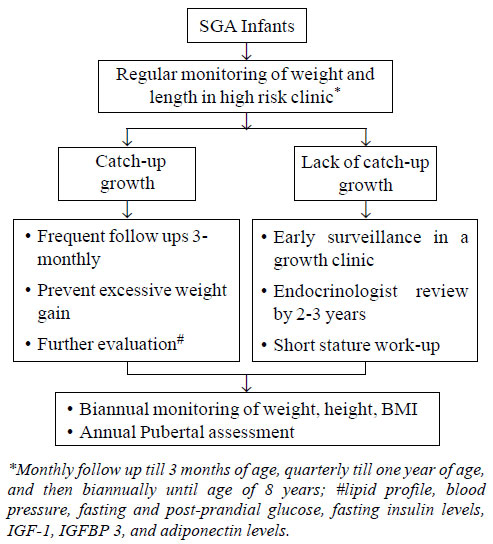 |
|
Fig. 1 Recommendations for follow up
of SGA infants.
|
Growth hormone (GH) therapy has been used in SGA
children with short stature with the aim of promoting growth, inducing
catch up to normal height early, reducing the psychosocial problems and
improving the social adaptation [17]. Intelligence and psychosocial
functioning have been shown to be enhanced during GH treatment [36].
Huisman, et al. [37] concluded that there is a positive
short-term effect of GH therapy on psychosocial functioning. It is
proposed that SGA children aged between 2-4
year who show no evidence of catch-up with a height less
than –2.5 SD should be
eligible for GH treatment. Intervention with GH for those with severe
growth retardation (height
SD score, <–2.5; age, 2-4 year) should
be considered at a dose of 35-70 µg/kg/day with higher
doses for the ones with marked growth
retardation.
The use of GH in short children born SGA has been officially approved
by the Food and Drug Administration in 2001 and by
the European Agency for the
Evaluation of Medicinal Products in 2003. Average height gain after 3
years of GH treatment range
from 1.2-2.0 SD for doses of 35-70
µg/kg/d. There should be a positive response to GH
treatment i.e. height
velocity SD score more than +0.5 in the first year of
treatment. In case of an
inadequate response, re-evaluation is indicated. GH treatment is
recommended till the growth
rate falls to less than 2 cm/year [2,3]. Prasad, et al. [38]
demonstrated a catch-up of +1.2 height SD score in SGA children with
height for age Z-score <-2.5 who received GH for 2 years, and it was not
associated with any significant adverse effects or acceleration of
puberty. However, the use of GH in resource-restrained setting is still
a matter of concern. Further, it remains to be determined whether GH
therapy in short children who were born SGA has any beneficial or
deleterious effect on their risk of developing metabolic syndrome in
adulthood. GH therapy has been shown to have no effect on onset of
puberty, progression of puberty, age at menarche and the interval
between the onset of breast development and menarche. Although GnRH
analog treatment might reduce growth velocity, evidence suggests that
combined GH and GnRH analog treatment may improve adult height in SGA
children who are short at the start of puberty (<140 cm) and have a poor
adult height expectation, and they also need higher GH dose [39].
Insulin sensitizer therapy has been proposed as
potentially beneficial for SGA girls with early-onset puberty. Ibanez,
et al. [40] published the effect of 36 months of Metformin
therapy for SGA girls with early-onset breast development and it was
found to be associated with slower pubertal development (prolonged time
span between B2 and menarche), prolonged pubertal height gain and
increased near-adult height. It was also associated with relatively
lower insulin, leptin and IGF-I levels and higher sex hormone-binding
globulin and IGFBP-1 levels, as well as a less atherogenic lipid profile
and leaner body composition.
References
1. Alkalay AL, Graham Jr JM, Pomerance JJ.
Evaluation of neonates born with intrauterine growth retardation:
review and practice guidelines. J Perinatol. 1998;18:142-51.
2. Clayton PE, Cianfarani S, Czernichow P,
Johannsson G, Rapaport R, Rogol A. Management of the child born
small for gestational age through to adulthood: A Consensus
Statement of the International Societies of Pediatric Endocrinology
and the Growth Hormone Research Society. J Clin Endocrinol Metab.
2007;92:804-10.
3. Lee PA, Chernausek SD, Hokken-Koelega AC,
Czernichow P. International Small for Gestational Age Advisory Board
Consensus Development Conference Statement: Management of the short
child born small for gestational age. Pediatrics. 2001;111:1253-61.
4. Karlberg J, Albertsson-Wikland K. Growth in
full-term small for gestational age infants: from birth to final
height. Pediatr Res. 1995;38:733-9.
5. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A,
Golan A. Morbidity and mortality among very-low-birth-weight
neonates with intrauterine growth restriction. The Vermont Oxford
Network. Am J Obstet Gynecol. 2000;182:198-206.
6. Saenger P, Czernichow P, Hughes L, Reiter E.
Small for gestational age: Short stature and beyond. Endocrine Rev.
2007;28:219-51.
7. Barker DJ, Osmond C, Forse´n TJ, Kajantie E,
Eriksson JG. Trajectories of growth among children who have coronary
events as adults. N Engl J Med. 2005; 353: 1802-9.
8. Verkauskiene R, Petraitiene I,
Albertsson-Wikland K. Puberty in children born small for gestational
age. Horm Res Paediatr. 2013;80:69-77.
9. Rapaport R, Tuvemo T. Growth and growth
hormone in children born small for gestational age. Acta Paediatr.
2005;94:1348-55.
10. Bhargava SK, Ramji S, Srivastava V, Sachdev
HPS, Kapani V, Datta V. Growth and sexual development of low birth
weight children: A 14 year follow up. Indian Pediatr.
1995;32:963-70.
11. Kushwaha KP, Singh YD, Bhatia VM, Gupta Y.
Clinical assessment of nutritional status (CANS) in term newborns
and its relation to outcome in neonatal period. J Neonatol.
2004;18:1.
12. Mehta S, Tandon A, Dua T, Kumari S, Singh SK.
Clinical assessment of nutritional status at birth. Indian Pediatr.
1998;35:423-8. National Neonatal-Perinatal Database, NNPD Network.
Indian Council of Medical Research. 2002-2003:25. Available from: http:/ www. newbornwhocc.org/ pdf/nnpd_report_2002-03.
Accessed
October 15, 2014.
13. Narang A, Chaudhari MK, Kumar P. Small for
gestational age babies: Indian scene. Indian J Pediatr.
1997;64:221-4.
14. Soto N, Bazaes RA, Pena V, Salazar T, Avila
A, Iniguez G, et al. Insulin sensitivity and secretion are
related to catch-up growth in small-for-gestational-age infants at
age 1 year: results from a prospective cohort. J Clin Endocrinol
Metab. 2003;88:3645-50.
15. Chellakooty M, Juul A, Boisen KA, Damgard IN,
Kai CM, Schimdt IM, et al. A Prospective study of serum
insulin like growth factor (IGF-1) and IGF binding protein-3 in 942
healthy infants: associations with birth weight, gender, growth
velocity and breast feeding. J Clin Endocrinol Metab. 2006;91:820-6.
16. Hokken-Koelega AC, De Ridder MA, Lemmen RJ,
Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born
small for gestational age: Do they catch up? Pediatr Res.
1995;38:267-71.
17. Albertsson-Wikland K, Boquszewski M, Karlberg
J. Children born small-for-gestational age: Postnatal growth and
hormonal status. Horm Res. 1998;49:7-13.
18. Chaudhari S, Otiv M, Hoge M, Pandit A, Mote
A. Growth and sexual maturation of low birth weight infants at early
adolescence. Indian Pediatr. 2008;45:191-8.
19. Rustogi D. To estimate Insulin like growth
factor-1 (IGF-1) and insulin levels in small for gestational age
(SGA) at the age of 12-18 months [MD thesis]. India: University of
Delhi; 2011.
20. Gupta A. evaluation of serum adiponectin
levels at 15-18 months of age amongst those born term small for
gestational age [MD thesis]. India: University of Delhi; 2013.
21. Tanaka T, Suwa S, Yokoya S, Hibi I. Analysis
of linear growth during puberty. Acta Paediatr Scand Suppl.
1988;347:25-9.
22. Deng HZ, Deng H, Su Z, Li YH, Ma HU, Chen HS,
et al. Insulin resistance and adiponectin levels are
associated with height catch-up growth in pre-pubertal Chinese
individuals born small for gestational age. Nutr Metab. 2012;9:107.
23. Ibanez L, Potau N, Zampolli M, Rique S,
Saenger P, Carrascosa A. Hyperinsulinemia and decreased insulin-like
growth factorbinding protein-1 are common features in prepubertal
and pubertal girls with a history of premature pubarche. J Clin
Endocrinol Metab. 1997;82:2283-8.
24. Jaquet D, Deghmoun S, Chevenne D, Czernichow
P, Levy-Marchal C. Low serum adiponectin levels in subjects born
small for gestational age: impact on insulin sensitivity. Int J Obes.
2006;30:83-7.
25. Neville KA, Walker JL. Precocious pubarche is
associated with SGA, prematurity, weight gain, and obesity. Arch Dis
Child. 2005;90:258-61.
26. Ong K, Kratzsch J, Kiess W, Dunger D.
Circulating IGF-I levels in childhood are related to both current
body composition and early postnatal growth rate. J Clin Endocrinol
Metab. 2002;87:1041-4.
27. Hokken-Koelega AC. Timing of puberty and
fetal growth. Best Pract Res Clin Endocrinol Metab. 2002;16:65-71.
28. Leger J, Levy-Marchal C, Bloch J, Pinet A,
Chevenne D, Porquet D, Collin D, Czernichow P. Reduced final height
and indications for insulin resistance in 20-year-old born small for
gestational age: regional cohort study. BMJ. 1997;315:341-7.
29. Sloboda DM, Hart R, Doherty DA, Pennell CE,
Hickey M. Age at menarche: influences of prenatal and postnatal
growth. J Clin Endocrinol Metab. 2007;92:46-50.
30. Ibanez L, Jimenez R, de Zegher F. Early
puberty- menarche after precocious pubarche: relation to prenatal
growth. Pediatrics. 2006;117:117-21.
31. Persson I, Ahlsson F, Ewald U, Tuvemo T,
Qingyuan M, von Rosen D, et al. Influence of perinatal
factors on the onset of puberty in boys and girls: implications for
interpretation of link with risk of long term diseases. Am J
Epidemiol. 1999;150:747-55
32. Barker DJ, Hales CN, Fall CH, Osmond C,
Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes
mellitus, hypertension and hyperlipidaemia (syndrome X): relation to
reduced fetal growth. Diabetologia. 1993;36:62-7.
33. Jaquet D, Deghmoun S, Chevenne D, Collin D,
Czernichow P, Levy-Marchal C. Dynamic change in adiposity from fetal
to postnatal life is involved in the metabolic syndrome associated
with reduced fetal growth. Diabetologia. 2005;48:849-55.
34. Ibanez L, Potau N, Enriquez G, Marcos MV, de
Zegher F. Hypergonadotrophinaemia with reduced uterine and ovarian
size in women born small-for-gestational-age. Hum Reprod.
2003;18:1565-9.
35. Ibanez L, de Zegher F. Puberty after prenatal
growth restraint. Horm Res. 2006; 65:112-5.
36. Van Pareren YK, Duivenvoorden HJ, Slijper FS,
Koot HM, Hokken-Koelega AC. Intelligence and psychosocial
functioning during long-term growth hormone therapy in children born
small for gestational age. J Clin Endocrinol Metab. 2004;
89:5295-302.
37. Huisman J, Slijper FM, Sinnema G, Akkerhuis
GW, Brugman- Boezeman AT. Good things come in small packages?
Psychosocial aspects of small stature. Tijdschr Kindergeneeskd.
1992;60:139-46.
38. Prasad HK, Khadilkar VV, Chiplonkar SA,
Khadilkar AV. Growth of Short Children born small for gestational
age and their response to growth hormone therapy. Indian Pediatr.
2013;50:497-9.
39. Lem AJ, van der Kaay DC, de Ridder MA,
Bakker-van Waarde WM, van der Hulst FJ, Mulder JC, et al.
Adult height in short children born SGA treated with growth hormone
and gonadotropin releasing hormone analog: results of a randomized,
dose-response GH trial. J Clin Endocrinol Metab. 2012;97:4096–105.
40. Ibanez L, Valls C, Ong K, Dunger DB, de Zegher F. Metformin
therapy during puberty delays menarche, prolongs pubertal growth, and
augments adult height: a randomized study in low-birth-weight girls with
early normal onset of puberty. J Clin Endocrinol Metab. 2006;91:2068-73.
|
|
|
 |
|

