|
|
|
Indian Pediatr 2011;48:
111-117 |
 |
Neonatal Zinc Supplementation for Prevention
of Mortality and Morbidity in Breastfed Low Birth Weight
Infants: Systematic Review of Randomized Controlled Trials |
|
Anjana Gulani, Shinjini Bhatnagar* and HPS
Sachdev
From the Department of Pediatrics and Clinical
Epidemiology, Sitaram Bhartia Institute of Science and Research, B-16
Qutab Institutional Area; and *Centre for Diarrheal Diseases Research and
Nutrition, Division of Gastroenterology, Hepatology and Nutrition,
Department of Pediatrics, All India Institute of Medical Sciences, Ansari
Nagar, New Delhi, India.
Correspondence to: Prof HPS Sachdev, E-6/12 Vasant Vihar,
New Delhi 110 057, India.
Email: hpssachdev@gmail.com
|
Objectives: To evaluate whether zinc supplements prevent mortality
and morbidity in breastfed low birth weight infants.
Methods: All randomized or qausi–randomized trials
with individual or cluster allocation and using concurrent controls were
included. Study population included LBW infants irrespective of
gestational status who were exclusively or predominantly breastfed at the
initiation of intervention. Intervention comprised zinc salts given as
tablets or syrups orally to provide at least 1 RDA of elemental zinc for
at least a period of 14 days, introduced within one month of birth.
Electronic databases were searched irrespective of language and
publication status.
Findings: Three trials from developing countries
met the inclusion criteria. Limited data did not indicate a reduced risk
of mortality (1 trial, RR=1.11; 95% CI 0.57 to 2.18 at one year),
hospitalization rate (1 trial, odds ratio 1.10; 95% CI 0.87 to 1.39),
acute respiratory infection (1 trial), or diarrhea (2 trials). However,
the trial reporting on mortality was not adequately powered for evaluating
this outcome. There was no evidence of an increase in weight (3 trials) or
height (2 trials) at either 6 months or one year of age, or of an
increased risk of vomiting following zinc supplementation. Serum zinc
levels at the end of intervention were significantly higher in the
supplemented group (2 trials).
Conclusions: In view of no convincing evidence of
benefits from the limited data available, currently there is no
justification for recommending routine zinc supplementation for breastfed
LBW newborns in developing countries.
Key words: Breastfed, Low birth weight, Morbidity, Mortality,
Neonate, Zinc.
|
|
Observational data indicates that higher
dietary zinc may be required to meet the daily requirements of low birth
weight breastfed infants [1-5]. In view of the important role of zinc in
immune function, it is conceivable that zinc supplementation in LBW
breastfed infants may prevent infectious morbidity and related mortality.
To inform policy, we conducted a systematic review of randomized
controlled trials to evaluate the effect of zinc supplementation on
mortality and morbidity in breastfed low birth weight infants.
Methods
Types of trials: All randomized or qausi–randomized
trials with individual or cluster allocation and using concurrent
controls. Trials employing a factorial design with multiple intervention
groups were eligible for inclusion.
Participants: Low birth weight infants (birth
weight less than 2500 grams) irrespective of gestational status who were
exclusively or predominantly breastfed at the initiation of intervention.
Exclusive breast-feeding was defined as no feed other than breastmilk.
Predominant breastfeeding was defined as taking only water or
multivitamins or medicines other than breastfeeds.
Intervention: Zinc salts given as tablets or syrups
orally to provide at least 1 RDA of elemental zinc [6, 7] for at least a
period of 14 days and introduced within one month of birth. Trials
providing additional supplements (for example, vitamin A, micronutrient
mixtures, iron) were considered if the only difference between the two
comparison arms was zinc supplement. The comparison groups included no
intervention or placebo.
Outcome measures
Primary: We examined all cause mortality in the
child at two time points: during infancy, in the period between initiation
of intervention and the last follow-up until the age of one year; and
during the neonatal period between initiation of intervention and the last
follow-up until the age of one month.
Secondary: In the period between initiation of
intervention and the last follow-up until the age of one year we measured
cause specific mortality due to diarrhea, acute respiratory infections and
causes other than these (as defined by the authors, irrespective of
ascribing a single or multiple causes of death), severity of morbidities
as assessed by clinic or hospital visits or hospitalizations (as defined
by the authors of the trials) and morbidities because of sepsis including
probable or culture proven bacterial sepsis, acute respiratory infection
or respiratory difficulty, diarrhea, meningitis, ear infections, cough or
running nose, fever or severe malnutrition (severe wasting, and pedal
edema or kwashiorkor). We also measured weight and height at the end of
intervention and adverse effects including vomiting.
Search Strategy
The trials were identified by simultaneous searches of
medical databases (till August 26, 2009) including PubMed (since 1966),
EMBASE (since 1980), Cochrane Controlled Trials Register, Web of Science (WoS),
Allied and Complementary Medicine (AMED) (since 1985), British Nursing
Index (BNI) (since 1994) and CAB abstracts (since 1973) with no language
restrictions. For PubMed the search strategy employed was: (newborn OR
neonat* OR infant OR neonates OR postnatal OR post-natal) AND (low birth
weight OR preterm OR small for gestational age OR premature) AND ("zinc"
OR micronutrient* OR vitamin*) AND ((clinical[Title/Abstract] AND
trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical
trial[Publication Type] OR random*[Title/Abstract] OR random
allocation[MeSH Terms] OR systematic OR review OR metaanalysis OR
meta-analysis).
A lateral search using the related articles link in
PubMed was done for selected articles. We reviewed the reference lists of
identified articles and hand searched reviews, and abstracts of
international micronutrient conferences of past three years. To avoid
publication bias, we included published and unpublished trials. Requests
for information were sent to experts and major development and aid
agencies including United Nations Children’s Fund (UNICEF), WHO, United
States Agency for International Development, and Bill and Melinda Gates
Foundation.
Data extraction and management: We extracted data
in duplicate on study design, participant characteristics, interventions,
and outcomes and contacted authors for additional information if required.
Any discrepancies in extracted data were resolved by discussion.
Assessment of risk of bias in included studies:
This was assessed in relation to sequence generation, allocation sequence
concealment, blinding, incomplete outcome data assessment, selective
reporting and any other bias [8].
Statistical Analysis
In factorial trials and in multi-arm designs yielding
two or more intervention groups (different dosage or administration
regimens) and a single control group, the data in the intervention groups,
including the variation in the intervention characteristic, was to be
pooled and compared against the single control group to prevent
unit-of-analysis error.
Data entry and initial analysis were performed on SPSS
(Version 13.0) software. Meta-analysis and meta-regression was to be
performed with user written programmes on Stata (version 9.2) software.
Presence of bias in the extracted data was to be evaluated by funnel plot
[9]. We were to use formal statistical tests for funnel plot asymmetry (Begg’s
and Egger’s) with the "metabias" command [10,11]. Pooled estimates
(relative risk with 95% confidence intervals) of the evaluated outcome
measures were calculated by the generic inverse variance method by "metan"
command [10,12]. For continuous outcomes (for example, weight and height)
pooled weighted mean differences (WMD) were computed using both fixed
effects and random effects models. We used formal tests of heterogeneity,
namely, the statistic Cochran Q and I 2
(variation in pooled estimate
attributable to heterogeneity) [13]. If I2 exceeded 25% and P value
for Cochran Q statistic was below 0.05, then heterogeneity was to be
considered substantial. In this eventuality, random effects model was to
be preferred and reasons for heterogeneity were to be explored by subgroup
analyses and meta-regression, if sufficient number of trials were
available.
Subgroup and sensitivity analyses: We were to
perform subgroup analyses only for the primary outcome, all cause
mortality within one year of age, as a hypothesis generating exercise. The
pre-specified subgroup analyses were to include: (i) dose of zinc;
(ii) duration of supplementation; (iii) birth weight; (iv)
gestational age (term gestation
³37
weeks versus preterm gestation <37 weeks); (v) follow-up age
to examine the possibility of a greater response in the first half of
infancy; (vi) infant mortality rate in the placebo group to examine
the possibility of a greater response with higher baseline mortality
[lower versus upper half (median) of infant mortality rate in
included trials]; (vii) Maternal zinc supplementation (yes vs
no); and (viii) development status of the trial area to examine the
possibility of a greater response in high-risk populations (developing
versus developed countries). We were also to conduct a sensitivity
analysis to investigate the robustness of results taking into account the
trial quality components (randomization, allocation concealment, blinding
and attrition). The contribution of these variables to heterogeneity was
to be explored by meta-regression with the restricted maximum likelihood
option [14].
Results
Using the search strategy, 12 potentially eligible
reports were identified [15-26]. Amongst these, 9 reports [15-23] were
excluded for various reasons (Web Fig. 1). The remaining 3
reports [24-26] provided data on 3 trials satisfying the inclusion
criteria. The baseline characteristics of the included trials, all from
developing countries [27] and the notable individual study specific
features are summarized in Web Table I
and Web Annexure.
Web Table
II summarizes the assessed risk of bias in the included studies. In 2
trials, no risk of bias was evident; while in the third trial; intention
to treat analysis was not performed. Data was insufficient to calculate
the routine measures of publication bias (Begg-Mazumdar bias and Horbold-Egger
bias statistics).
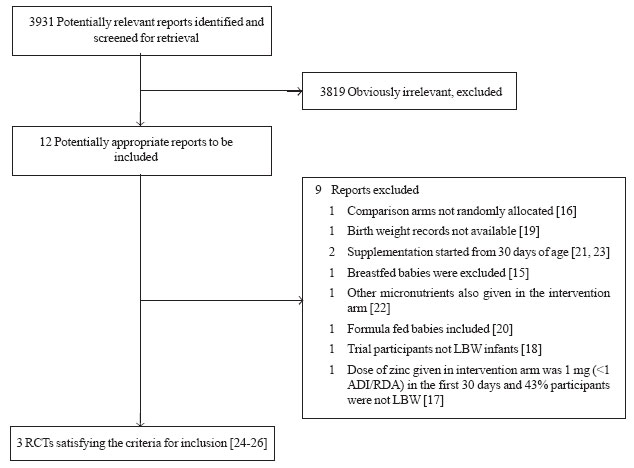 |
|
Fig. 1 Flow chart depicting the
trial flow for selection of randomized control trials included in
the systematic review. |
Assessment of heterogeneity: As the search
retrieved only 3 trials with information on outcomes of interest being
available in even lesser trials, the estimate of I 2
statistic should not be considered
to be robust (Table I). Most of the evaluated outcomes
suggested evidence of significant heterogeneity (I2 or Cochran Q
statistics). We therefore preferred to use the random effects model
estimates.
Table I
Summary of Pooled Estimates for Various Outcome Measures
|
Outcome |
No. of |
Random effects model |
Fixed effects model |
Tests for heterogeneity |
|
|
trials |
RR/WMD^ (95% CI); |
RR/WMD^ (95% CI); |
I2 (%); Q (P value) |
|
|
|
P value |
P value |
|
|
Diarrhea till 1 year |
2 |
0.87 (0.65, 1.16); 0.337 |
0.96 (0.89, 1.03); 0.211 |
70.1; 3.34 (0.067) |
|
Diarrhea till 1 year* |
2 |
0.97 (0.90, 1.04); 0.375 |
0.97 (0.90, 1.04); 0.375 |
0.0; 0.10 (0.751) |
|
Weight (z scores) at 6 mo^ |
3 |
0.13 (-0.18, 0.45); 0.412 |
-0.01 (-0.10, 0.08); 0.868 |
64.4; 5.62 (0.06) |
|
Weight (z scores) at 1 year^ |
3 |
0.42 (-0.20, 1.04); 0.183 |
0.01 (-0.08, 0.10); 0.801 |
90.6; 21.6 (<0.001) |
|
Length (cm) at 6 mo^ |
2 |
-0.21 (-0.41, 0.00); 0.052 |
-0.21 (-0.41, 0.00); 0.052 |
0.0; 0.48 (0.487) |
|
Length (cm) at 1 year^ |
3 |
0.48 (-1.47, 2.43); 0.629 |
-0.09 (-0.32, 0.15); 0.487 |
90.1; 20.3 (<0.001) |
|
Serum zinc (microgram/dL) |
2 |
18.45 (2.39, 34.52); 0.024 |
15.58 (11.41, 19.75); <0.001 |
92.1; 12.7 (<0.001) |
* With number of children instead of number of episodes for the Sur, et al. [34] trial.
|
Primary Outcomes
Infant mortality: Only one trial reported on the
number of infant deaths [26]. Of the 1026 infants randomized in each
group, there were 21 deaths in the supplemented and 19 deaths in the
placebo groups. The relative risk of mortality with zinc supplementation
group was 1.11 (95% CI 0.57 to 2.18) at one year.
Neonatal mortality: No trial reported on the number
of neonatal deaths.
Secondary outcomes
Cause specific mortality: The only trial [26] with
information on deaths did not report on cause specific mortality.
Severity of morbidities: Only one trial [26]
reported on hospitalization rate as number/1000 child-years of follow up
(189.9 in zinc supplemented and 175.3 in placebo groups, respectively).
The odds ratio for hospitalization with zinc supplementation was 1.10 (95%
CI 0.87 to 1.39). No trial had reported upon clinic or hospital visits.
Acute respiratory infection (ARI) or respiratory
difficulty: One trial reported [26]
on ARI at 3, 6, 9 and 12 months as
morbidity recall within the past 24 hours or within the past 7 days. There
was no evidence of reduced ARI with zinc supplementation for both these
measures at any time point of evaluation. The pooled relative risk (random
effects model) of reported ARI within 7 days was 1.13 (95% CI 0.98 to 1.3)
till one year of age and 1.13 (95% CI 0.96 to 1.34) till 6 months of age.
Diarrhea: Two trials from India [25,26]
had reported on diarrhea. There was no evidence of a reduced risk of
diarrhea with zinc supplementation (Table I). The trial from
Kolkata, India [25] had only reported on the number of diarrheal episodes
without adjustment for individuals having more than one episode. On
replacing the number of diarrheal episodes with the number of individuals
developing diarrhea in this trial [25], there was no evidence of a reduced
risk of diarrhea (RR 0.97; 95% CI 0.90 to 1.04). The Kolkata trial [25]
had also reported on the proportion of days ill with diarrhea/child/year,
which was comparable during the exclusive breast feeding period (zinc
vs placebo 3.7 vs 4.0; P>0.05) but significantly lower
in the zinc supplemented group during the post breastfeeding period (6.6
vs 10.2; P<0.0001).
Other morbidities: No trial reported morbidities
other than diarrhea and ARI.
Growth: Three trials provided information on
weight. There was no evidence of a greater weight for age z scores
following zinc supplementation either at 6 months or one year of age (Table
I). Two trials [25,26]
provided information on height. There was
no evidence of a greater height (cm) following zinc supplementation either
at 6 months or one year of age (Table I).
Adverse effects: Only one trial had reported on
adverse effects [26]. There was no evidence of an increase in vomiting
following zinc supplementation. The pooled relative risk (random effects
model) of vomiting with zinc supplementation was 1.06 (95% CI 0.88 to
1.28) till one year of age and 1.17 (95% CI 0.91 to 1.51) till 6 months of
age.
Serum zinc levels: Two trials [24, 26] had
evaluated serum zinc levels at the end of the trial. Data were available
from 387 participants (186 in zinc group and 201 in placebo group). The
serum zinc levels ( mg/dL)
were significantly higher following zinc supplementation (Table
I).
Exploratory subgroup and meta-regression analyses were
not feasible because outcomes were reported in a small number (one to
three) of trials.
Discussion
There was no convincing evidence of benefit following
zinc supplementation in breastfed LBW infants. Limited data from 1 to 3
trials did not indicate a reduced risk of mortality (1 trial),
hospitalization rate (1 trial), acute respiratory infection (1 trial), or
diarrhea (2 trials). However, the trial reporting on mortality was not
adequately powered for evaluating this outcome. There was no evidence of
an increase in weight (3 trials) or height (2 trials) at either 6 months
or one year of age or of an increased risk of vomiting following zinc
supplementation. Serum zinc levels at the end of intervention were
significantly higher in the supplemented group (2 trials) indicating
successful absorption of the micronutrient.
Following limitations merit consideration. First, only
three trials satisfied the inclusion criteria and inferences on some
outcomes were based on 1 or 2 trials only. The only trial reporting on
mortality was not adequately powered to evaluate this outcome. Second, all
trials were conducted in developing countries and two were from India,
which limits the generalization of findings to other regions in developing
countries and developed nations. Third, the possibility of publication
bias and predictors for heterogeneity could not be explored due to the
small number of trials. Fourth, information on exclusive or predominant
breast feeding status was available only at the time of initiation of the
study but not in a longitudinal manner till the end of the intervention.
The following criterion for data inclusion deserves
elucidation: (i) The intervention was evaluated in LBW breastfed
infants to guide policy formulation by the WHO for optimal feeding of LBW
infants in developing countries. LBW newborns comprise a heterogeneous
population of preterm and small for gestational age (SGA) newborns that
are physiologically different. Preterm infants are likely to have higher
zinc deficit and dietary requirements as nearly 60% fetal zinc is acquired
during third trimester of pregnancy. It is therefore conceivable that the
response to zinc supplementation may be variable amongst preterm and SGA
babies. It would therefore have been ideal to evaluate the effect of
intervention in relation to gestation and growth retardation. However,
most of the relevant trials have not segregated the data into preterm
and/or growth retarded babies. Also in the context of developing
guidelines in resource starved countries, the only practical option is
categorization as LBW rather than SGA and/or preterm because gestation is
difficult to determine with accuracy. Further, nearly 70% of LBW babies in
developing countries are SGA unlike in the developed world where the bulk
of LBW babies are preterms [1]. Thus we feel that our study criteria are
apt to feed the policy requirement. (ii) The type of intervention
was defined as zinc salts given as tablets or syrups orally to provide at
least 1 RDA of elemental zinc for at least a period of 14 days introduced
within one month of birth. The RDAs for zinc in low birth weight breast
fed infants have not been defined [6, 7]. However, the suggested average
dietary intakes (ADI, which are invariably lower than RDA) for infants in
the age group of 0 to 6 months are 2 mg(6) and it is believed that the
requirements would be higher in LBW infants. We therefore used the
conservative cut-off of 2 mg elemental zinc intake to define the RDA in
LBW newborns.
One trial [17]
had provided information on mortality but was excluded because the zinc
dose was 1 mg (<1 ADI/RDA) in the first 30 days of life and 43% of the
participants were full-term small for gestational age but not low birth
weight (>2500 grams). We could not include the data relevant to our
systematic review from this trial because the report did not present
disaggregated results for LBW infants. Also, despite supplementation
commencing at 15 days of age, effect on mortality was reported at 1 to 9
months of age. In this factorial design trial, the multivariate analysis
using Cox regression (5 deaths in 581 zinc supplemented participants
versus 15 deaths in 573 participants not receiving zinc) yielded a
relative risk of 0.32 (95% CI 0.12 to 0.89; P=0.028). Stratified
results for low birth weight participants were not available. On including
this trial also in the pooled estimates, there was no evidence of a
reduced risk of mortality in infancy (Web Fig
1); the pooled RR was 0.63 (95% CI 0.19 to 2.13; P=0.458; I2=75.5%
with P= 0.043 for heterogeneity) by random effects model and 0.76
(95% CI 0.43 to 1.32; P=0.324) by fixed effects model.
It could be postulated that beneficial effects were not
evident because the intervention may not have led to an increase in zinc
nutriture of the participants. However, a significant increase of serum
zinc in sub-groups of two trials refutes this hypothesis. Further, as per
the inclusion criteria the participants had received at least one
recommended ADI per day.
All the reviewed evidence pertains to developing
countries, and is primarily from populations at risk of developing zinc
deficiency. In view of no convincing evidence of benefits from the limited
data available currently, there is no justification for recommending
routine zinc supplementation for breastfed low birth weight newborns in
these populations. Future research and trials on this subject should
examine: outcomes in more settings in Asia and Africa; and should be
adequately powered to estimate mortality. These trials should also record
feeding status of participants and record causes of death and morbidities
other than diarrhea and ARI.
Contributors: AG prepared the protocol, applied the
search strategy, retrieved the articles, and extracted data. HPSS
developed the idea for review, finalized the protocol and search strategy,
confirmed the extracted data, and did the statistical analysis. SB
prepared and finalized the protocol. All authors contributed to the
drafting of the final version of the paper. AG and HPSS will act as joint
guarantors.
Funding: Department of Child and Adolescent Health
and Development, World Health Organization and Sitaram Bhartia Institute
of Science and Research, B-16 Qutab Institutional Area, New Delhi, India.
The funding sources had no involvement in the study or the decision to
publish the manuscript.
Competing interest: None stated.
References
1. Sachdev HPS, Shah D. Epidemiology of maternal and
fetal malnutrition in South Asia. In: Bhutta ZA, editor. Oxford:
Perinatal and Newborn Care in South Asia. Ed.: Oxford University Press.
2007. p. 75-105.
2. Singh PP, Khushlani K, Veerwal PC, Gupta RC.
Maternal hypozincemia and low-birth-weight infants. Clin Chem.
1987;33:1950.
3. Goel R, Misra PK. Study of plasma zinc in neonates
and their mothers. Indian Pediatr. 1982;19:611-4.
4. Jeswani RM, Vani SN. A study of serum zinc levels in
cord blood of neonates and their mothers. Indian J Pediatr. 1991;58:683-6.
5. Bhandari N, Taneja S, Mazumder S, Bahl R, Fontaine
O, Bhan MK; Zinc Study Group. Adding zinc to supplemental iron and folic
acid does not affect mortality and severe morbidity in young children. J
Nutr. 2007;137: 112-7.
6. Dietary reference intakes for Vitamin A, Vitamin K,
Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum,
Nickel, Silicon, Vanadium, and Zinc. A Report of the Panel on
Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and
of Interpretation and Uses of Dietary Reference Intakes, and the Standing
Committee on the Scientific Evaluation of Dietary Reference Intakes, Food
and Nutrition Board, Institute of Medicine. Zinc. National Academy Press,
Washington, DC, 2001 p 442-501. Available from: www.nap.edu/openbook.php?record_id=
10026 & page=442. Accessed on December 2, 2009.
7. Hotz C. Dietary indicators for assessing the
adequacy of population zinc intakes. Food Nutr Bull. 2007;28:S430-48.
8. Higgins JPT, Altman DG on behalf of the Cochrane
Statistical Methods Group and the Cochrane Bias Methods Group. Assessing
risk of bias in included studies. In: Higgins JPT, Green S, eds.
Cochrane Handbook for Systematic Reviews of Interventions. New Jersey:
John Wiley & Sons Inc. 2008. p.187-241.
9. Sterne JAC, Egger M, Smith GD. Investigating and
dealing with publication and other biases. In: Egger M, Smith GD,
Altman DG, eds. Systematic Reviews in Health Care: Meta-analysis in
Context. London: BMJ Books. 2001. p. 189-208.
10. Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in
STATA TM. In: Egger M, Smith GD, Altman DG editors. Systematic
Reviews in Health Care: Meta-analysis in Context. London: BMJ Books. 2001.
p.347-369.
11. Steichen TJ, Egger M, Sterne JAC. sbe19.1:
Tests for publication bias in meta-analysis. Stata Technical
Bulletin. 1998;44:3-4.
12. Harris R, Bradburn MJ, Deeks J, Harbord R, Altman
D, Steichen T, et al. Stata Version 9 Update (Distribution Date
February 19, 2007) for the Stata User Written Programme sbe24 (Bradburn MJ,
Deeks JJ, Altman D. sbe24: metan - an alternative meta-analysis
command. Stata Technical Bulletin 1999; 44: 4-15). Available from:
http://fmwww. bc.edu/RePEc/bocode/m. Accessed on March 24, 2007.
13. Higgins JPT, Thompson SG. Quantifying heterogeneity
in a meta-analysis. Stat Med. 2002;21:1539-58.
14. Harbord R, Steichen T, Sharp SJ. 2004. "metareg:
update to Stata module to perform meta-analysis regression" (Sharp SJ.
sbe23: Meta-analysis regression. Stata Technical Bulletin 1998; 42:
16-22). Statistical Software Components S4446201, Boston College
Department of Economics, revised 02 Feb 2005. Available from: http: //ideas.repec.org/c/boc/bocode/s446201.html.
Accessed March 15, 2007.
15. Friel JK, Andrews WL, Matthew JD, Long DR, Cornel
AM, Cox M, et al. Zinc supplementation in very-low-birth-weight
infants. J Pediatr Gastroenterol Nutr. 1993;17:97-104.
16. Ashworth A, Morris SS, Lira PI, Grantham-McGregor
SM. Zinc supplementation, mental development and behaviour in low birth
weight term infants in northeast Brazil. Eur J Clin Nutr. 1998;52:223-7.
17. Sazawal S, Black RE, Menon VP, Dinghra P, Caulfield
LE, Dhingra U, et al. Zinc supplementation in infants born small
for gestational age reduces mortality: a prospective, randomized,
controlled trial. Pediatrics. 2001;108:1280-6.
18. Castillo-Durán C, Perales CG, Hertrampf ED, Marín
VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and
growth of Chilean infants. J Pediatr. 2001;138:229-35.
19. Osendarp SJM, Santosham M, Black RE, Wahed MA, van
Raaij JMA, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo of
life on growth and morbidity of Bangladeshi infants in urban slums. Am J
Clin Nutr. 2002;76:1401-8.
20. Díaz-Gómez NM, Doménech E, Barroso F, Castells S,
Cortabarria C, Jiménez A. The effect of zinc supplemen-tation on linear
growth, body composition, and growth factors in preterm infants.
Pediatrics. 2003;111:1002-9.
21. Black MM, Sazawal S, Black RE, Khosla S, Kumar J,
Menon V. Cognitive and motor development among small-for-gestational-age
infants: impact of zinc supplementation, birth weight, and caregiving
practices. Pediatrics. 2004;113:1297-1305.
22. Loui A, Raab A, Wagner M, Weigel H,
Grüters-Kieslich A, Brätter P, et al. Nutrition of very low birth
weight infants fed human milk with or without supplemental trace elements:
a randomized controlled trial. J Pediatr Gastroenterol Nutr.
2004;39:346-53.
23. Jimenez R, Martinez M, Penalver R. Effect of zinc
on growth and development of children with low birth weight. Colombia
Medica. 2007;38:6-13.
24. Castillo-Duran C, Rodriguez A, Venegas G, Alvarez
P, Icaza G. Zinc supplementation and growth of infants born small for
gestational age. J Pediatr. 1995;127:206-11.
25. Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B,
Rajendran K, et al. Impact of zinc supplementation on diarrheal
morbidity and growth pattern of low birth weight infants in Kolkata,
India: a randomized, double-blind, placebo-controlled, community-based
study. Pediatrics. 2003;112:1327-32.
26. Taneja S, Bhandari N, Rongsen-Chandola T,
Mahalanabis D, Fontaine O, Bhan MK. Effect of zinc supplementation on
morbidity and growth in hospital-born, low-birth-weight infants. Am J Clin
Nutr. 2009;90:385-91.
27. Human Development Report 2009, United Nations
Development Programme. Available from: http://hdr.undp.org/en/media/HDR_2009_EN_Complete.pdf.
Accessed on November 26, 2009.
|
|
|
 |
|

