|
Live
attenuated Hepatitis A vaccines derived from H2 strains were licensed for
human use in 1992. Since then, these vaccines have been used successfully
in the Chinese population in the primary prevention of Hepatitis A
infections as well as in the control of epidemics [1-5].
The first study of this vaccine outside China was
conducted at Pune, India in 2004, and showed an immunogenicity of 95.8% by
the end of 2 months after a single dose of the vaccine [6]. The high
immunogenicity of the single dose schedule in Indian children was
corroborated in 2008 by a larger multicentre study, showing seroconversion
of 95.1% and 97.9% at 6 weeks and 6 months, respectively [7]. The original
Pune cohort has been under regular follow up since vaccination, and this
report refers to the immunogenicity data of this cohort at 30 months after
vaccination.
Methods
All children who completed the first phase of the study
are called regularly to our centre for a follow up clinical visit once a
year. At each visit, the children are evaluated with: (i) detailed
medical history, especially enquiring about jaundice, and (ii)
physical examination including size of liver and spleen.
At 30 months (after vaccination), their blood samples
were collected and sent for estimation of anti-HAV IgG antibodies (HAVB
2.0; Abbott Axsym, ELISA) to an independent accredited laboratory (Super
Religare Laboratories, Mumbai). Seroprotection was defined as anti-HAV
antibody (IgG) level ³20mIU/mL
and immunogenicity was defined as the percentage of seroprotected
subjects. The study was conducted after the approval of the Institutional
Ethics Committee.
Results
Of the original 143 subjects, 131 came for the 30 month
immunogenicity study. Of the 12 ‘dropouts’ 9 have transferred to other
towns/ countries and 3 were refusals. Of the 131 subjects enrolled for
follow up study, 8 subjects with sampling errors were excluded from
immunogenicity analysis. Six of the remaining 123 subjects were ‘vaccine
failures‘ from initial study and hence antibody titers were analyzed in
117 subjects (73 boys) with a mean age of 7.2 ± 2.6 years (range 3.5 - 15
years).
The number of children seen at each yearly clinical
review were 139 (2005), 136 (2006), and 131 (2007). No reports of
hepatitis like illnesses were recorded in any of the subjects since
vaccination. 30 months after vaccination, protective antibody levels (³20
mIU/mL) persisted in 108 of the 117 evaluable subjects (92.3%). By
including the 6 vaccine failures from the Initial study, long term
immunogenicity over 30 months was calculated as 87.8%. The geometric mean
titre (GMT) of anti-HAV antibodies of all 117 evaluable subjects was 92.02
mIU/mL, while that of the 108 seroprotected subjects was 111.16 mIU/mL.
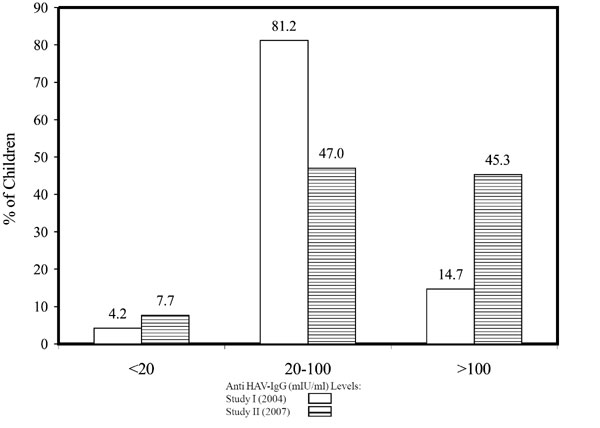
The distribution of the subjects as per their antibody titres in
comparison with the Initial study is seen in Fig. 1.
The number of subjects with titers >100 mIU/mL had increased significantly
in the Follow up study (P< 0.001).
 |
|
Fig. 1 Percent distribution of subjects in
the Initial study and the Follow up Study as per their antibody
titres. |
Discussion
The current follow up study demonstrates an
immunogenicity of 87.8% with a GMT of 92.02mIU/mL, 30 months after a
single dose of live attenuated Hepatitis A vaccine (Zhepu, Zhejiang Pukang
Biotechnological Company Ltd, China). The results compare well with long
term immunogenicity data following mass vaccination programs in various
centres in China [4,5,8]. The Shanghai study reported seroprotective
levels of 94.9% at 8 weeks falling to approx 80% by 3 years [4]. Cohort
studies by Zhuang, et al. [5] recorded seroprotective levels in
98.6 % at 2 months, falling to 83.3% at 6 years and 80.2% at 10 years with
GMTs of 287 mIU/mL, 173 mIU/mL and 145 mIU/mL, respectively.
Wang, et al. [9] compared immunogenicity data of
two doses of live versus two doses of inactivated hepatitis A vaccine (Havrix,
GSK Biologicals) at 12 and 24 months post vaccination. At 12 months, the
reported GMT levels were 448 mIU/mL for live vaccine versus 1063 mIU/mL
for inactivated vaccine whereas the corresponding values for 24 months
were 218 mIU/mL versus 655 mIU/mL, respectively. At 24 months,
seroprotection in this study was 92% for live vaccine and 100% for
inactivated vaccine. Although seroprotective levels and GMTs achieved by a
two dose schedule (inactivated or live attenuated vaccines) are somewhat
higher [8-10], the excellent efficacy of a single dose schedule in
prevention of symptomatic hepatitis A in epidemics has been demonstrated
convincingly [1-5]. It has been suggested that exposures to natural
infections (after vaccination) may act as ‘booster doses’, a phenomena of
considerable significance in developing countries like India, which are
endemic for Hepatitis A [8]. This may also explain the increased titres of
HAV antibodies in our study at 30 months despite no further vaccination.
There have been no cases of hepatitis A like illness in
study subjects till date, but the efficacy of the single dose schedule in
our children will be elucidated by the long term follow up of our cohort.
Acknowledgments: Dr Ganesh Kadhe, Dr Vinita
Satyavrat, Dr Rasendra Kumar Jha and Dr Bharat Mahajan for their
assistance and guidance in carrying out this study and the Medical social
workers at KEM Hospital for the follow-up.
Contributors: ShB and AB were responsible for
designing of the study. AS was primarily responsible for day to day
conduct of the study. AB carried out statistical analysis. ShB, AB and SB
coordinated the study and drafted the paper. AP supervised all aspects of
the study. ShB will act as guarantor for the manuscript.
Funding: Wockhardt India Ltd, Mumbai
Competing interests: Seema Bawangade is an employee
of Wockhardt India Ltd, Mumbai.
|
What This Study Adds?
•
Immunogenicity at 30 months
following single dose of live attenuated Hepatitis A vaccine in
Indian children is 87.8%.
|
References
1. Mao JS, Chai SA, Xie RY, Chen NL, Jiang Q, Zhu XZ,
et al. Further evaluation of the safety and protective efficacy of
live attenuated hepatitis A vaccine (H2 strain) in humans. Vaccine.
1997;15:944-6.
2. Zhuang F, Jiang Q, Gong Y. Epidemiological effects
of live attenuated hepatitis A vaccine (H(2)-strain): results of a 10-year
observation. Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:188-90.
3. Zhang Y, Liu X, Ma J. A field evaluation of the
epidemiological efficacy of an attenuated live hepatitis A vaccine (H2
strain). Zhonghua Yu Fang Yi Xue Za Zh. 2001;35:387-9.
4. Xu Z, Wang X, Li R,Meng Z, Zhang Y, Gong J, et al.
Immunogenicity and efficacy of two live attenuated hepatitis A vaccines
(H2 strain and LA-1 strains). Zhonghua Yi Xue Za Zhi. 2002;82:678-81.
5. Zhuang F, Qian W, Mao Z, Gong Y, Jiang Q, Jiang L,
et al. Persistent efficacy of live attenuated hepatitis A vaccine
(H2-strain) after a mass vaccination program. Chin Med J.
2005;118:1851-6.
6. Bhave S, Bavdekar A, Madan Z, Jha R, Bhure S,
Chaudhari J, et al. Evaluation of immnunogenicity and tolerability
of a live attenuated Hepatitis A vaccine in Indian children. Indian
Pediatr.2006;43:983-7.
7. Faridi MMA, Shah N, Ghosh TK, Sankaranarayanan, VS,
Arankalle V, Aggarwal A, et al. Immunogenicity and safety of live
attenuated Hepatitis A vaccine: A multicentric study. Indian Pediatr.
2009;46:29-34.
8. Wang XY, Xu ZY, Ma JC, Von Seidlein L, Zhang Y, Hao
ZY, et al. Long – term immunogenicity after single and booster dose
of a live attenuated hepatitis A vaccine: Results from 8- year follow-up.
Vaccine. 2007;25:446-9.
9. Wang XY, Xu Z, Yao X, Tian M, Zhou L, He L, et al.
Immune responses of anti-HAV in children vaccinated with live attenuated
and inactivated hepatitis A vaccines. Vaccine. 2004;22:1941-5.
10. Chan CY, Lee SD, Yu MI, Wang YJ, Chang FY, et al.
Long-term follow-up of hepatitis A vaccination in children. Vaccine.
1999;17:369-72.
|

