|
Sunil Kumar Gupta,
T.I. Khan,
R.C. Gupta,
A.B. Gupta,
K.C. Gupta,
Pradeep Jain and
Alka Gupta
From the Satellite Hospital, Banipark, Jaipur 302
006, India; Indira Gandhi Center for HEEPS, University of Rajasthan,
Jaipur 302 004, India; Department of Physiology and Human Fertility
Research Center, R.N.T. Medical College, Udaipur, India; Malaviya
Regional Engineering College, Jaipur 302 017, India; Public Health
Engineering Department and Departments of Dentistry and Physiology,
S.M.S. Medical College, Jaipur 302 004, India.
Correspondence to: Dr. Sunil Kumar Gupta, A
31-B, Anita Colony, Bajaj Nagar, Jaipur 302 015, India. E-mail:
[email protected]
Manuscript received: March 21, 2000, Initial review
completed: May 12, 2000,
Revision accepted: August 29, 2000.
Objective:
To evaluate the effect of varying
ingestion of drinking water containing high fluorides and its effect on
serum parathyroid hormone. Design: Cross sectional clinical
study. Setting: S.M.S. Medical College, Jaipur. Subject:
200 children were selected from four areas (50 from each area) consuming
water containing 2.4, 4.6, 5.6 and 13.5 mg/l of fluoride. All children
were in an age group of 6 to 12 years. Methods: All children were
graded for clinical, radiological and dental fluorosis and biochemical
estimations were made for serum calcium, serum and urinary fluoride and
serum parathyroid hormone. Results: Serum calcium levels were
well within normal range in the patients of all areas but an increase in
serum parathyroid levels (S. PTH) was noted. The increased S. PTH was
well correlated with increase in fluoride ingestion. The severity of
clinical and skeletal fluorosis was observed to increase with increase
in S. PTH concentration. Conclusions: High Fluoride ingestion has
a definite relationship with increased parathyroid hormone secretion,
which may be responsible for maintaining serum calcium levels and may
have a role in toxic manifestations of fluorosis.
Key words:
Calcium, Fluorosis, Parathormone.
SYSTEMIC
fluorosis is an
endemic problem in several developing countries especially in India and
Pakistan and has also been reported sporadically in other parts of the
world(1). While the WHO guidelines permit only 1.5 mg/L (ppm) as a safe
limit for human consumption(2), people in seventeen states of India are
consuming water with fluoride concentrations even up to 44 mg/L(3-5). In
many of these areas people still do not have any alternative but to
drink such water. Worse still, with the depletion of limited ground
water sources containing low fluoride, in some pockets more and more
people are forced to consume water rich in fluoride.
Toxic effect of excessive fluoride(1,6,7) take three
forms: clinical, skeletal and dental. General manifestations include
dental dis-coloration, dental as well as skeletal defor-mities, severe
joint pains, general debility and psychosocial problems due to bad
teeth, body deformities and immobility.
Various studies on human and animals were conducted
to evaluate the effect of fluoride ingestion on parathyroid hormones.
The results were contradictory, and largely inconclusive. Jenkins et
al.(8) reported nor-mal parathyroid function in cases of chronic
fluorosis. The studies reported by Jowsey et al. indicated that
high doses of fluoride result in a depression of serum calcium, causing
stimulation of parathyroid gland activity and increased release of the
hormone, and hence increase resorption of bone(9). Teotia et al.
in their study on static and dynamic histomorphometric measurements
revealed the profiles of osteomalacia and secondary hyperparathyroidism
in varying combinations in all cases(10). High fluoride ingestion
disturbs the calcium homeostasis and bone structure. The role of
parathyroid hormone in maintaining calcium homeostasis and bone
structure is well known. Therefore it was planned to evaluate the effect
of varying ingestion of high fluoride drinking water on serum
parathyroid hormone.
The aims of the study were explained to all the
patients and their parents. A written free and informed consent and an
authority to publish the results of the study and related photographs
were obtained from all of them. Requisite amount of blood was drawn for
the tests after they had given the consent.
Fifty children were selected randomly from each of
the 4 areas, based on the drinking water fluoride concentration as
follows:
Group A: Ramsagar ki Dhani (2.4 ppm)
Group B: Rampura (4.6 ppm)
Group C: Shivdaspura (5.6 ppm)
Group D: Raipuria (13.6 ppm)
The concentration of fluoride in drinking water was
measured using ion selective electrode method using Orionís pH/ISE
meter, model 920A(11). Twenty five milli liters of sample and 25 ml of
TISAB were mixed in a beaker. Electrode was rinsed with distilled water
and placed into beaker. The concentration was noted when "Rdy"
was displayed on the instrument.
All the children were in an age group of 6 to 12
years, their body weight ranging from 18 to 30 kg. These children were
graded for clinical (non skeletal), skeletal (radiological) and dental
fluorosis(10,12). The details are depicted in Table I.
|
Table I
- Grading
of Fluorosis
|
|
Clinical Grading(10) |
|
Grade I:
|
Mild - generalized bone and joint pain.
|
|
Grade II: |
Moderate - generalized bone and joint pain, stiffness
and rigidity, restricted movements at spine and joints.
|
|
Grade III: |
Severe - symptoms of moderate grading with
deformities of spine and limbs, knock knees, crippled or bedridden
state.
|
|
Grading of Skeletal Fluorosis(10) (Radiological
examination)
|
|
Grade I: |
Mild - osteosclerosis only.
|
|
Grade II: |
Moderate -
osteosclerosis, periosteal bone formation,
calcification of interosseous membrane, ligaments, capsules,
muscular attachments, tendons.
|
|
Grade III: |
Severe - findings as in moderate with
exostoses,
osteophytosis and associated metabolic bone disease.
|
|
Grading of Dental Fluorosis(12)
|
| Type
|
Grade
|
Description
|
|
Normal Enamel
|
0
|
The enamel presents the
usual translucent semi-vitriform type of structure. The
surface is smooth, glossy' and usually of a pale,
creamy-white color.
|
| Questionable
fluorosis
|
0.5
|
Slight
aberrations from the translucency of normal enamel seen;
ranging from a few white flecks to occasional white spots.
This classification is used in instances where a definite
diagnosis of the mildest form of fluorosis is not
warranted and a Classification of "Normal" not
justified.
|
Very
mild
fluorosis
|
1
|
Small opaque,
paper-white areas scattered irregularly over the tooth but
not involving as much as approximately 25% of the tooth
surface. Frequently included in this classification are
teeth showing no more than about 1-2 mm of white opacity
at the tip of the summit of the cusps of the bicuspids or
second molars.
|
| Mild fluorosis
|
2
|
The white opaque areas
in the enamel of the teeth are more extensive, but do not
involve as mush as 50% of the tooth.
|
Moderate
flurosis
|
3
|
All enamel surface
of the teeth are affected and surfaces subject to
attrition show marked wear. Brown stain is frequently a
disfiguring feature.
|
Severe
fluorosis
|
4
|
All enamel surface are affected and hypoplasia is
so marked that the general form of tooth may be affected. The major
diagnosis of this classification is the discrete or confluent pitting.
Brown stains are widespred, and teeth often present a corroded like
appearance.
|
Biochemical investigations included measurement of
levels of serum calcium, serum and urinary fluoride and serum
parathyroid hormone. Serum calcium was measured by OCPC method using kit
supplied by Wako, Japan(13). O-cresolphthalein com-plexone (OCPC)
combines with alkaline earth metals to assume a purplish red color. The
8 hydroxyquinoline in the color reagent affords color development of
calcium specifically. The calcium content of the sample can be
determined by measuring the absorbence at 570 nm. The density of the
purplish red color produced by OCPC is proportional to the calcium
content.
Mid molecule assay of Parathyroid hor-mone was done
by radioimmunoassay(14) using PTH-MMTM||125|
RIA Kit supplied by Incstar (Incstar Corporation - Stillwater Minnesota,
USA) with the sensitivity as the apparent concentration at 2 standard
devia-tions from the counts at maximum binding; the minimum detectable
amount was 9.8 pmol/L. The PTH-MM || RIA is a disequili-brium procedure
using delayed tracer addition to increase sensitivity. Antiserum is
directed to only the mid-region of human parathyroid hormone. Iodination
is done by conventional methods utilizing synthetic hPTH (Tyr43) 44-68.
In this RIA, sample and PTH-MM anti-serum are combined and incubated for
15 minutes at room temperature. Tracer is then added, followed by a
second incubation for 2 hours at 4 degree Celsius. Phase separation is
done in 15 minutes with a pre-precipitated complex of second antibody,
carrier, and PEG added in a single pipetting step. Standards are
expressed as picomoles/liter of mid-molecule fragment.
The urinary fluoride was measured using ion selective
electrode method(11). Twenty five ml of sample and 25 ml of TISAB were
mixed in a beaker. Electrode was rinsed with distilled water and placed
into beaker. Concentration was noted when "Rdy" was displayed
on the instrument.
Concentration of fluoride in serum was measured using
ion selective electrode method(11). Two ml of sample was mixed with 8 ml
of fluoride standard solution of 1 ppm and to this 10 ml of TISAB were
added and mixed in a beaker. Electrode was rinsed with distilled water
and placed into beaker. Concentration was noted when "Rdy" was
displayed on the instrument. The values of fluoride in serum were
calculated.
The observations related to grading of clinical,
dental and skeletal fluorosis are depicted in Table II. The
severity of dental fluorosis observed in these areas (represented in
terms of Deanís scale) was: Ramsagar ki dhani - 2.71, Rampura - 1.73
Shivdaspura - 2.44 and Raipuria 3.43. The severity of clinical and
skeletal fluorosis was almost same in Groups A and B. The clinical
mani-festations of clinical and skeletal fluorosis started rising in
children of Group C and increased abruptly in Group D.
|
Table II - Fluorosis
Grading in Subjects
|
|
Village
|
Dental fluorosis
Mean (SD)
|
Clinical (Non-skeletal) fluorosis Mean (SD) |
Skeletal (Radiological) fluorosis Mean (SD) |
|
Ramsagar ki Dhani
|
2.71(1.09)
|
0.95(0.22)
|
0.68(0.67)
|
|
Rampura
|
1.73(1.09)
|
1.00(0.00)
|
0.50(0.61)
|
|
Shivdaspura
|
2.44(1.32)
|
1.00(0.00)
|
0.79(0.91)
|
|
Raipuria
|
3.43(1.70)
|
1.51(0.51)
|
0.95(1.12)
|
|
|
|
|
The biochemical parameters (Table III)
indicate that there was an increase in serum parathyroid levels (S. PTH)
with increasing fluoride ingestion. The serum and urinary fluoride
concentrations were also higher. It was also observed that out of the
total fluoride intake through water and food, drinking water was the
major source. The serum calcium levels were well within normal range in
all areas.
|
Table III - Biochemical
Parameters in Subject
|
|
Village
|
S.PTH-MM II Mean(SD) (pmol/l)
|
S. calcium Mean(SD) (mg/dl)
|
Serum fluoride mean(SD)
(mg/dl)
|
Urinary fluoride mean(SD) (mg/dl)
|
Drinking water fluoride (mg/L) |
Fluoride through water mean (SD) (mg/dl) |
Flouoride
through food mean(SD) (mg/dl) |
Total fluoride intake (food and water) mean(SD) (mg/dl)
|
|
Ramasagar ki Dhani
|
31.64
|
9.23
|
0.79
|
9.45
|
2.4
|
5.00
|
2.45
|
7.35
|
| |
(2.82)
|
(1.89)
|
(0.21)
|
(4.11)
|
|
(1.11)
|
(1.47)
|
(1.72)
|
|
Rampura
|
40.98
|
10.75
|
1.10
|
15.90
|
4.6
|
9.71
|
2.07
|
11.97
|
| |
(26.9)
|
(1.66)
|
(0.58)
|
(9.98)
|
|
(2.23)
|
(1.00)
|
(1.8)
|
|
Shivdaspura
|
75.07
|
9.68
|
1.10
|
17.78
|
5.6
|
12.04
|
2.41
|
14.45
|
| |
(31.75)
|
(0.99)
|
(0.17)
|
(7.77)
|
|
(2.78)
|
(0.65)
|
(3.19)
|
|
Raipuria
|
125.10
|
10.39
|
1.07
|
14.56
|
13.6
|
30.26
|
2.30
|
32.56
|
| |
(131.14)
|
(1.44)
|
(0.17)
|
(7.88)
|
|
(9.52)
|
(0.82)
|
(9.33)
|
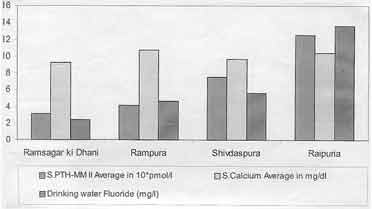
S. PTH levels showed an increasing trend with
increasing fluoride ingestion through drinking water fluoride
concentration. The serum calcium was within the normal range in all
groups (Fig. 1). There was a high positive correlation (r =
0.967) between S. PTH and fluoride concentration in drinking water.
Fig.
1. Drinking water fluoride, serum calcium and serum PTH-MM II in
different areas
The observations indicated a definite trend of
increase in severity of dental fluorosis with increasing fluoride
ingestion. The higher severity of dental fluorosis at Ramsagar ki dhani
among all the areas (even though the fluoride concentration in drinking
water and total daily intake was the lowest among all four selected
areas), can possibly be explained by poor dental hygiene indicated by
high prevalence of dental caries (76%) in this area during this study
whereas it was only 10% in village B, 8% in village C and 12% in village
D.
Earlier workers (9,15) reported that fluoride and PTH
have a definite role in bone metabolism. Studies have documented that
ingestion of fluoride causes decrease in the ionic calcium (8,16,17). An
increase in PTH along with decrease in ionised calcium after isoflurane
inhalation has been observed (18). Srivastava et al.(19) observed
significantly elevated PTH concetration in the presence of normal, total
and ionized calcium con-centrations.
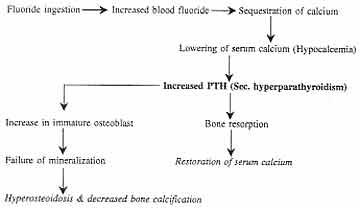
The hypocalcemia caused by high fluoride ingestion
leads to changes in internal milieu of the body to maintain the
calcium levels and causes secondary hyperparathyroidism (Fig. 2).
Lowering of blood ionized calcium by an amount as low as 0.02 mmol/l
within 30 min elicited an immediate large, transient peak release of PTH
amounting to 6-16 times the baseline concentration(20).
|
|
|
Fig.
2. Flow chart showing a possible mechanism of secondary
hyperparathyroidism due to high fluoride ingestion |
|
.
This secondary hyperparathyroidism results in two
effects(17):
(a) Maintenance of serum calcium: An
increase in serum calcium concentration is always the consequence of
at least one of the following events: (a) an increase in the
net calcium input in extracellular fluid, (b) a decrease in
glomerular filtration rate, and (c) an increase in the tubular
re-absorption of the filtered calcium. The parathyroid helps in
maintaining the calcium balance mainly by inducing tubu-lar calcium
reabsorption and mobilization from bone(21).
(b) An increased bone resorption, defective
bone formation and defective collagen (ground substance) formation
(8,9,16,17).
The observations indicated that in Groups A and B,
the levels of S. PTH were well within normal range (48.1 Ī 11.9 pmol/L),
whereas in Groups C and D the levels went beyond normal range, probably
due to rela-tively greater quantity of ingested fluoride. In view of the
observations made by Gupta et al.(17), the increased S. PTH
secretion might be responsible for the more severe mani-festations of
clinical and skeletal fluorosis in children of Groups C and D.
It would be prudent to state an important limitation
of this study. We were unable to estimate the vitamin D levels.
In conclusion, high fluoride ingestion causes
secondary hyperparathyroidism, which may be responsible for maintaining
serum calcium levels and may play a role in causing toxic manifestations
of fluorosis.
The help rendered by the Santokhba Durlabhji Memorial
Hospital in conducting the PTH estimation is gratefully acknow-ledged.
Contributors: SKG
was principal coordinator of the study and will act as the guarantor.
TIK and ABG helped in environmental designing of the field study; RCG
carried out the biochemical analysis and helped in interpretation of
data and drafting of the paper with ABG; KCG helped in data handling; PJ
helped in grading dental fluorosis; and AG carried out all field work.
Competing interests:
None stated.
Funding:
Department of Science and
Technology, Government of Rajasthan, India.
-
Susheela A.K. Pevention and Control of Fluorosis:
Technical information for Training cum Awareness Camp for Doctors,
Public Health Engineers and other Officers, New Delhi, National
Technology Mission of Drinking Water. 1991.
-
World Health Organization. Guidelines for
Drinking Water Quality, Geneva, Volume 2. World Health Organization,
1984; p 249.
-
Thergaonkar VP, Bhargava RK. Water Quality and
incidence of fluorosis in Jhun-jhunu District of Rajasthan:
Preliminary observations. Indian J Env Hlth, 1974; 16:168-180.
-
Choubisa SL, Sompura K, Bhatt SK, Choubisa DK,
Pandya H, Joshi SC, Prevalence of Fluorosis in some villages of
Dungarpur District of Rajasthan. Indian J Hlth 1996; 38: 119-126.
-
Public Health Engineering Department. Fluo-ride
affected villages: Habitat survey Rajas-than, PHED, Rajasthan, Jaipur
1991-93, pp 1-21.
-
World Health Organization, Geneva, WHO Monograph
Series No. 59, 1970.
-
World Health Organization, Geneva, Fluo-rine and
Fluoride, Geneva, World Health Organization. 1984; p 93.
-
Jenkins GN, Venkateswarlu P, Zipkin I.
Physiological effects of small doses of fluoride. In: Fluoride
and Human Helath, Geneva. World Health Organization, 1970; pp 163-223.
-
Jowsey I, Riggs BL, Kelly PJ. Long term
experience with fluoride and fluoride combination treatment of
osteoporosis. In: Calcium Metabolism, Bone and Metabolic Bone
Diseases: Proceedings of the X European Symposium on Calcified
Tissues, Hamburg (Germany), 16-21 September, Eds Cordt FK, Kruse HP.
Berlin, Springer-Verlag, 1975; pp 151-154.
-
Teotia SPS, Teotia M, Singh DP. Bone static and
dynamic histomorphometry in endemic fluorosis. In: Fluoride
Research 1985: Studies in Environmental Science, Vol 27, Amster-dam,
Elsevier Science Publishers, 1985; pp 347-355.
-
Fuchs C, Dom D, Fuchs CA, Henning HV, Meintosh
C, Scheler F. Fluoride deter-mination in plasma by ion selective
electrode: A simplified method for the clinical labo-ratory. Clin Chim
Acta 1975; 60: 157-167.
-
Dean HT. Classification of mottled enamel
diagnosis. J Am Dent Assoc 1934; 21: 1421-1426.
-
Connerty VH, Briggs RA. Determination of serum
calcium by means of orthocresol-phthalein complexone. Am J Clin Path
1966; 45: 290-296.
-
Lindall AW, Ells JE, Roos B. Estimation of
biologically active intact parathyroid hor-mone in normal and
hyperparathyroid sera by sequential N-terminal immunoextraction and
midregion radiommunoassay. J Clin Endo-crinol Meta 1983; 57:
1007-1014.
-
Armstrong WD, Messer H, Singer L. Effect of
bone fluoride on bone resorption and metabolism. In: Friedrich
Kuhlen cordit and Hans Peter Kruse. Calcium Metabolism, Bone and
Metabolic Bone Diseases. Proceedings of the X European Symposium on
Calcified Tissues, Hamburg (Germany), 16-21 Septem-ber 1973. Eds. Cord
FK, Kruse HP, Berlin, Springer-Verlag, 1975; pp 132-133.
-
Teotia SPS, Teotia M. Hyper activity of the
parathyroid glands in endemic osteofluorosis, Fluoride, 1972, 5:
115-126.
-
Gupta SK. Environmental Health Perspective of
Fluorosis in Children, Ph.D. Thesis, University of Rajasthan, Jaipur,
Rajasthan, 1999.
-
Hotchkiss CE, Brommage R, Du M, Jerome
CP. The anesthetic isoflurane decreases ion-ised calcium and increases
parathyroid hor-mone and osteocalcin in cynomolgus monkeys. Bone 1998;
23: 479-484.
-
Srivastava RN, Gill DS, Moudgil A, Menon RK,
Thomas M, Dandona P. Normal ionised Calcium. Parathyroid
hypersecretion, and ele-vated osteocalcin in a family with fluorosis.
Metabolism, 1989; 38: 120-124.
-
Schwartz P. Madsen JC, Rasmussen AQ, Transbol
IB, Brown EM. Evidence for a role of intracellular stored parathyroid
hormone in producing hysteresis of the PTH-Calcium relationship in
normal humans. Clini Endo-crinol 1998; 48: 725-732.
- Houlillier-P, Blanchard-A, Paillard IV. Extra-parathyroid
hypercalcemia: Physiopatho-logical and therapeutic aspects. Ann Med
Interne Paris, 1997; 148: 15-18.
|
