|
|
|
Indian Pediatr 2013;50:
1113-1118 |
 |
Aeroallergen Sensitization in Childhood
Asthmatics in Northern India
|
|
Dinesh Raj, Rakesh Lodha, Anibha Pandey, Aparna
Mukherjee, Anurag Agrawal, *SK Kabra, and
New Delhi Childhood Asthma Study Group
From Division of Pediatric Pulmonology, Department of
Pediatrics, All India Institute of Medical Sciences, Ansari Nagar, New
Delhi 110029; *Institute of Genomics and integrative Biology, Mall Road,
Delhi.
Correspondence to Dr Rakesh Lodha, Department of
Pediatrics, All India Institute of Medical Sciences,
New Delhi, India 110029.
Email:
[email protected]
Received: February 11, 2013;
Initial review: March 09, 2013;
Accepted: July 03, 2013;
Published online: July 5, 2013.
PII: S097475591200141
|
Objective: To determine the prevalence of sensitization to common
aeroallergens in asthmatic children and study the differences in
characteristics of atopics and non atopics.
Design: Analysis of data from a prospective
cohort study.
Setting: Pediatric Chest Clinic of tertiary care
center in Northern India
Patients: Asthmatic children from 5-18 year of
age.
Main outcome measures: Prevalence of
sensitization to common aeroallergens.
Results: Skin prick testing (SPT) was performed
on 180 children above 5 years of age, with a mean (SD) age of 111.4
(34.2) months. 100 children (55.6%) were sensitized to at least one
aeroallergen, suggesting atopy; 68 (37.8%) were sensitized to more than
one allergen. 36.7% children were sensitized to housefly antigen; 31.1%
to rice grain dust, 18.3% to cockroach, and 7.8% to house dust mite
antigens. Atopic children had significantly higher median FENO during
follow up than non-atopic children (17.5 ppb vs 13 ppb, P=0.002).
There was a positive correlation between age and the number of allergens
that an individual was sensitized to (r= 0.21; P=0.0049).
Conclusions: More than half of asthmatic children
in our cohort had sensitization to one or more aeroallergens suggesting
atopy; sensitization was most commonly seen to housefly antigen and rice
grain dust. Atopic children had significantly higher FENO measurements
during follow up as compared to non-atopic children.
Key words: Aeroallergens, Atopy, Skin prick testing.
|
|
A
topic (or allergic) asthma is a phenotype of
asthma, which is characterized by allergic sensitization. Children with
atopic asthma typically have onset early in life, have a positive family
history of asthma or allergy, may have other coexistent allergic
diseases (atopic dermatitis, allergic rhinitis), produce IgE specific to
identifiable allergens, and have asthma exacerbation triggered by these
allergens [1].
Aeroallergen sensitization is a risk factor in the
development of childhood asthma, and most commonly implicated allergens
are house dust mite (HDM), cockroach, and furred animals. Aeroallergen
sensitization can be evaluated using either skin testing or measuring
specific IgE to these aeroallergens. Skin prick testing (SPT) is an
easy, cost-effective and convenient approach to identify sensitization
to allergens. SPT detects the presence of allergen specific IgE bound to
mast cells by eliciting mast cell degranulation to the specific allergen
being tested [2].
Children with allergen sensitization are likely to
have severe asthma, poorer lung function, and severe exacerbation due to
these allergens [3,4]. The purpose of this study was to determine the
prevalence of sensitization to common aeroallergens and study the
differences in characteristics of atopics and non-atopics.
Methods
This study was conducted at a tertiary care center in
northern India. We are following a cohort of pediatric asthma patients
(up to 18 yrs) from the Pediatric Chest Clinic since August 2009.
Eligibility criteria were children with asthma who stayed in Delhi and
nearby areas and willing to follow up 3 monthly regularly for at least 1
year. The diagnosis and treatment of asthma was based on the Global
Initiative for Asthma (GINA) guidelines [5]. At enrolment, baseline data
was collected, spirometry was performed [6], and FENO measurement was
done [7]. Asthma was classified as per the NAEPP guidelines [8]. The
patients were followed up every 3 months, symptom diary was maintained,
and control was assessed as per GINA guidelines [5]. The cohort included
243 asthmatic children. As SPT needs cooperation of the child, we
performed SPT on children who were above five years of age. Of the
cohort of 243 children, 180 children were 5 or more years of age and
gave consent to SPT.
SPT was done using 12 aeroallergens. Saline was taken
as negative control and histamine was used for positive control.
Patients were not on antihistamines for at least 48 hours preceding the
test. The twelve allergens tested were rice grain dust, wheat threshing
dust, housefly, dog dander, female cockroach, house dust mite (Dermatophagoides
farinae), Curvularia lunata, Aspergillus tamari,
Alternaria tenius, Prosopis juliflora, Cynodon dactylon,
and Holoptelea integrifolia. Allergens were obtained from
All Cure Pharma Pvt Ltd, Bahadurgarh, Haryana. As it was not feasible
for us to use a large panel, we used aero-allergens which we suspected
to be of relevance considering our patient population (urban, rural,
urban slum) and other previous studies from our country. Test was
considered positive if wheal in any of the allergens was 3 mm or more
than the negative control. Child was considered atopic if he
demonstrated positive result to one or more allergen, and non-atopic if
he had a negative SPT.
Study protocol was approved by Institutional Ethics
committee of All India Institute of Medical Sciences.
Statistical analysis: Data were entered
using Microsoft Access. Statistical analysis was performed using Stata
9.0 statistical software (Stata Corp., College Station, TX, USA).
Differences between normally distributed variables were tested using
unpaired Student’s t-test whereas non-normally distributed variables
were tested with two – sample Wilcoxon rank-sum (Mann-Whitney) test.
Chi-square test was used for testing difference in proportions for
categorical variables. P value of <0.05 was considered
significant.
Results
Skin prick testing was performed on 180 children
above 5 years of age (75.6% males), with a mean age 9.2 ± 3.0 years.
Baseline characteristics of the cohort are given in Table I.
One hundred children (55.6%) were sensitized to at least one
aeroallergen, suggesting atopy. Of the sensitized children, 49% had
moderate persistent asthma, 38% had mild persistent asthma, 12% had
severe persistent asthma, and 1% had intermittent asthma. The most
common prevalence of aeroallergen sensitization was to housefly (36.7%)
(Table II).
TABLE I Baseline Characteristics
|
Characteristics |
n (%) |
|
Baseline asthma severity
|
|
|
Intermittent asthma, n (%) |
4 (2.2)
|
|
Mild persistent asthma, n (%) |
61 (33.9)
|
|
Moderate persistent asthma, n (%) |
96 (53.3)
|
|
Severe persistent asthma, n (%) |
19 (10.6) |
|
Age of onset of symptoms (n=177) |
35.0 (35.7) |
|
Exposure to tobacco smoke at home, (%) |
70 (39.6) |
|
Atopy (skin prick testing, n=180) |
|
|
Negative, n (%) |
80 (44.4) |
|
Positive to at least one allergen, n (%) |
100 (55.6) |
|
Positive to more than one allergen, n (%) |
68 (37.8) |
|
Residence |
|
|
Rural, n (%) |
43 (24.0) |
|
Urban, n (%) |
130 (72.6) |
|
Urban slum, n (%) |
6 (3.4) |
|
Family history of asthma, n (%) |
85 (47.8) |
|
Family history of any allergic disease |
112 (62.9) |
TABLE II Aeroallergens Causing Sensitization
|
Aeroallergen |
n (%) |
Age, mean (SD) |
|
Housefly |
66 (36.7) |
124 (34.3) |
|
Rice grain dust |
56 (31.1) |
115.6 (35.2) |
|
Female cockroach |
33 (18.3) |
116.5 (35.4) |
|
Curvularia lunata |
27 (15.0) |
113.7 (30.0) |
|
Aspergillus tamari |
26 (14.4) |
125.1 (30.1) |
|
Alternaria tenius |
18 (10.0) |
119 (32.2) |
|
Dog dander |
14 (7.8) |
119.3 (34.2) |
|
House dust mite |
14 (7.8) |
123.9 (37.5) |
|
Cynodon dactylon |
12 (6.7) |
127.6 (38.0) |
|
Holoptelea integrifolia |
12 (6.7) |
124.3 (31.6) |
|
Wheat threshing dust
|
12 (6.7) |
120.3 (35.4) |
|
Prosopis juliflora |
3 (1.7) |
131.3 (45.2) |
TABLE III Comparison between Atopic and Non-atopic Asthmatics
|
Atopic (n=100) |
Non-atopic (n=80) |
P value |
|
Mean age, years (SD) |
9.8 (2.9) |
8.3 (2.9) |
0.0009 |
|
Males, n (%) |
80 (80) |
56 (70) |
0.121 |
|
Mean weight, kg (SD) |
29.1 (10.1) |
22.4 (8.5) |
<0.0001 |
|
Mean height, cm (SD) |
132.0 (19.6) |
122.1 (29.1) |
0.007 |
|
Residence |
|
|
|
|
Urban, n (%) |
25 (22.8) |
18 (25.0) |
0.796 |
|
Urban slum, n (%) |
71 (74.7) |
59 (71.0) |
|
|
Rural, n (%) |
4 (2.5) |
2 (4.0) |
|
|
Baseline asthma severity |
|
|
|
|
Intermittent, n (%) |
1 (1) |
3 (3.8) |
0.276 |
|
Mild persistent, n (%) |
38 (38) |
23 (28.8) |
|
|
Moderate persistent, n (%) |
49 (49) |
47 (58.8) |
|
|
Severe persistent, n (%) |
12 (12) |
7 (8.8) |
|
|
Family history of asthma, n (%) |
42 (42.4) |
43 (54.4) |
0.111 |
|
Family history of asthma or allergy, n (%) |
58 (58.6) |
54 (68.4) |
0.186 |
|
Baseline FEV1, % predicted, mean (SD) |
85.2 (19.8) n=92 |
85.8 (17.8) n=65 |
0.826 |
|
Baseline PEF, % predicted, mean (SD) |
74.5 (22.2) n=92 |
68.2 (22.2) n=69 |
0.076 |
|
Baseline FENO, median (IQR), ppb |
18 (10-30) n=91 |
14 (10-21.5) n=64 |
0.052 |
|
Mean FENO during follow up, median (IQR), ppb |
17.5 (11.7-27.5) n=93 |
13 (9.7-17.5) n=77 |
0.002 |
|
Mean FENO during exacerbation, median (IQR), ppb |
18 (13-25.5) n=42 |
14 (10-22) n=27 |
0.202 |
|
Number of acute exacerbations per child per year, mean
(SD) |
0.86 (1.63) n=95 |
0.63 (1.26) n=77 |
0.319 |
|
Age of onset of symptoms (mo), mean (SD) |
39.2 (40.0) |
29.3 (28.7) |
0.059 |
|
Daily dose of inhaled corticosteroids (µg), mean (SD) |
580.0 (226.2) |
573.0 (224.7) |
0.841 |
The characteristics of atopics and non-atopics are
given in Table III. 155 children out of 180 were able to
perform FENO appropriately as per guidelines [7]. There was a tendency
towards lower median FENO at baseline in the non-atopic group (P=0.052).
During follow up, median FENO was significantly lower in the non atopic
group (P=0.002); however, this difference was not observed during
acute exacerbation. There was a positive correlation between age and the
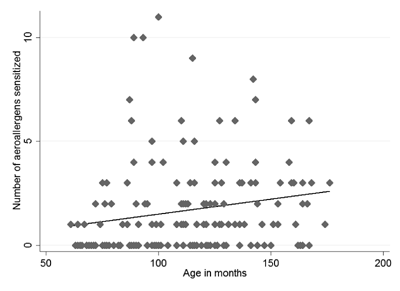
number of allergens that an individual was sensitized to (r= 0.21; P=0.0049)
(Fig. 1).
 |
|
Fig. 1 Correlation between age and the
number of aeroallergen sensitized.
|
Discussion
In our study, 55.6% children of our cohort were
sensitized to one or more aeroallergens. Atopy and allergen exposure are
known to exacerbate asthma and atopy is a risk factor for relapse of
asthma after remission [9]. Prevalence of atopy (defined as at least one
positive SPT) in childhood asthmatics varies from 45 to 79% and
percentage of asthma cases attributable to atopy in population based
studies varies from 25 to 63% [10].
We used a panel of 12 aeroallergens for skin testing.
Sensitization to housefly and rice grain dust was the commonest. There
are few studies which have evaluated insect sensitization in asthma
patients [11-13]. Rice grain dust is also an uncommon cause of
respiratory allergy [14]. Although these are infrequent cause of
allergic sensitization, it won’t be prudent to dismiss them as just
irritation than sensitization.
The sensitization pattern observed in our study is
different from other studies from the same geographical region. An
earlier study from North India which assessed 480 asthmatics/allergics
found Prosopis juliflora among pollen and Alternaria
alternata as important sensitizers with 34.7% and 17.7% skin
positivity, respectively [15]. Another study from Southern India in
patients with nasobronchial allergy showed high prevalence of mite
allergy (73.7%) and pollen allergy (75.8%) [16]. The reason for this
difference from same geographical area is probably because of seasonal
and annual fluctuations in allergens. Heterogeneity in allergen extract
composition can possibly lead to the different pattern of sensitization
observed in our study [17]. The variable composition and content of
allergenic extract of different manufacturers may affect the
allergenicity of the extract [18].
Sensitization to house dust mite has been
incriminated in the development of asthma, and has been observed in over
50% children and adolescents [19]. Sensitization to allergens (mite, dog
or cat) in the first 3 years of life is associated with loss of lung
function at school age [20]. A whole population birth cohort study
identified house dust mite as the most common allergen [21]. Dust mite
allergy has also been associated with increased asthma morbidity and
severity [22]. Sensitization to house dust mite was observed in 7.8%
patients in our cohort. The low incidence of sensitization to house dust
mite in our study is surprising given the high prevalence of
sensitization in studies from the developed countries. There is limited
data on house dust mite allergy in asthmatic children in India. A study
from Mysore in children and adults with allergic rhinitis and/or asthma
found dust mite allergy in 65-70% [23].
Our study showed a positive correlation between age
and the number of allergens that an individual was sensitized to. In a
recent birth cohort study, it was seen that allergen sensitization
continued to increase over childhood and adolescence, and presence of
sensitization at 4 years predicted later sensitization to additional
allergens [24].
Evidence on the relation between asthma severity and
sensitization or atopy is conflicting [25-28]. In our study, asthma
severity was not different between atopics and non-atopics. This can be
explained by the fact that acute exacerbation and loss of asthma control
can be caused by a number of factors other than allergen exposure.
Non-atopic asthmatics were younger than atopic
asthmatics, and had a trend towards younger age of onset of asthma.
Similar findings have been reported from a hospital based study from
Spain (29). Other characteristics like ICS use, acute exacerbation
episodes per child per year, and PFT measurements were similar in the
two groups.
Allergen sensitization has been known to cause
increased FENO, not only in children but also adults [30-32]. FENO has
been found to be elevated not only in asthmatics but also other atopic
conditions like allergic rhinitis and atopic eczema. Atopic individuals
have allergic inflammation and the allergological markers (BAL
eosinophils, blood eosinophils, eosinophils in bronchial biopsies) are
known to correlate with FENO. The exact reason for high FENO in
eosinophilic inflammation is not clear, however there could be a
possible role of NOS 2 upregulation. Similarly, in our study, FENO was
significantly higher in atopic children as compared to non atopic
children at follow up (P=0.002). However, there was no difference
in FENO at baseline and during acute exacerbation in the two groups. The
reason why the difference at baseline was not observed may be because of
the fact that at enrolment the patients were heterogeneous in terms of
asthma control and steroid use. This study corroborates the fact that
atopic asthmatics have higher FENO, but whether this helps to identify a
clinically relevant phenotype of asthma, has to be studied [33].
Few studies are available from the Indian
subcontinent which have evaluated prevalence of allergic sensitization
in childhood asthma and also FENO in relation to allergic sensitization.
The cohort is being followed up to evaluate the natural history of
atopic asthma and allergic sensitization. One limitation of our study is
that the panel of aero-allergens used may have been inadequate and a
larger panel should be used to thoroughly evaluate the aero-allergens in
our geographic region. The study was conducted in a hospital and not in
the general population, so inherent sampling bias cannot be ruled out
and therefore, our results may not be generalizable. In subjects with a
negative SPT, further additional panels of allergens were not tested and
therefore, true non-atopy could not be confirmed.
The pattern of alloallergen sensitization in this
study is not similar to earlier studies from this or other geographical
regions. Atopic children had significantly higher FENO measurements as
compared to non-atopic children. There is a need of know about an
optimal panel of aeroallergens which can be only done by studies using a
larger panel of aeroallergens.
Contributors: AA, RL and SKK conceived and
designed the study, analyzed the data and were directly involved in
paper writing; RL will act as guarantor; DR, AP, were responsible for
data collection; DR and AM contributed to analysis and drafting of the
paper; New Delhi childhood asthma study group participated in
development of study.
Funding: None; Competing interests: None
stated.
New Delhi Childhood Asthma Study Group: Meenakshi
Nagarkar, Bipin Jose, Anirban Sinha, Tarpritesh Sethi, Balaram Ghosh.
References
1. Wenzel SE. Asthma phenotypes: the evolution from
clinical to molecular approaches. Nat Med. 2012;18:716-25.
2. Carr TF, Saltoun CA. Chapter 2: Skin testing in
allergy. Allergy Asthma Proc. 2012;33:S6-8.
3. Langley SJ, Goldthorpe S, Craven M, Morris J,
Woodcock A, Custovic A, et al. Exposure and sensitization to
indoor allergens: association with lung function, bronchial reactivity,
and exhaled nitric oxide measures in asthma.J Allergy Clin Immunol.
2003;112:362-8.
4. Sala KA, Carroll CL, Tang YS, Aglio T, Dressler
AM, Schramm CM. Factors associated with the development of severe asthma
exacerbations in children.J Asthma. 2011;48:558-64.
5. From the Global Strategy for Asthma Management and
Prevention, Global Initiative for Asthma (GINA) 2011. Available from:
http://www.ginasthma.org/.
6. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi
R, Coates A, et al. ATS/ERS Task Force. Standardisation of
spirometry. Eur Respir J. 2005;26:319-38.
7. American Thoracic Society; European Respiratory
Society. ATS/ERS recommendations for standardized procedures for the
online and offline measurement of exhaled lower respiratory nitric oxide
and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005 Apr
15;171:912-30.
8. National Asthma Education and Prevention Program.
Expert Panel Report: Guidelines for the Diagnosis and Management of
Asthma. Bethesda, Md: US Dept of Health and Human Services, Public
Health Service; April 1997. NIH publication 97-4051.
9. Taylor DR, Cowan JO, Greene JM, Willian AR, Sears
MR. Asthma in remission: can relapse in early adulthood be predicted at
18 years of age? Chest. 2005;127:845-50.
10. Pearce N, Pekkanen J, Beasley R. How much asthma
is really attributable to atopy? Thorax. 1999;54:268-72.
11. Smith TS, Hogan MB, Welch JE, Corder WT, Wilson
NW. Modern prevalence of insect sensitization in rural asthma and
allergic rhinitis patients. Allergy Asthma Proc. 2005;26:356-60.
12. Focke M, Hemmer W, Wöhrl S, Gotz M, Jarisch R,
Kofler H. Specific sensitization to the common housefly (Musca domestica)
not related to insect panallergy. Allergy. 2003;58:448-51.
13. Lierl MB, Riordan MM, Fischer TJ. Prevalence of
insect allergen-specific IgE in allergic asthmatic children in
Cincinnati, Ohio. Ann Allergy. 1994;72:45-50.
14. Kayaba H, Meguro H, Muto H, Kamada Y, Adachi T,
Yamada Y, et al. Activation of eosinophils by rice-husk dust
exposure: a possible mechanism for the aggravation of asthma during rice
harvest. Tohoku J Exp Med.2004;204:27-36.
15. Sharma S, Kathuria PC, Gupta CK, Nordling K,
Ghosh B, Singh AB. Total serum immunoglobulin E levels in a casecontrol
study in asthmatic/allergic patients, their family members, and healthy
subjects from India. Clin Exp Allergy. 2006;36:1019-27.
16. Lal A, Sunaina Waghray S, Nand Kishore NN. Skin
prick testing and immunotherapy in nasobronchial allergy: our
experience. Indian J Otolaryngol Head Neck Surg. 2011;63:132-5.
17. Casset A, Mari A, Purohit A, Resch Y, Weghofer M,
Ferrara R, et al. Varying allergen composition and content
affects the in vivo allergenic activity of commercial Dermatophagoides
pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253-62.
18. Esch RE. Allergen source materials and quality
control of allergenic extracts. Methods. 1997;13:2-13.
19. Ulrik CS, Backer V. Markers of impaired growth of
pulmonary function in children and adolescents. Am J Respir Crit Care
Med. 1999;160:40-4.
20. Illi S, von Mutius E, Lau S, Niggemann B, Gruber
C, Wahn U. Multicentre Allergy Study (MAS) group. Perennial allergen
sensitisation early in life and chronic asthma in children: a birth
cohort study. Lancet. 2006;368:763-70.
21. Arshad SH, Tariq SM, Matthews S, Hakim E.
Sensitization to common allergens and its association with allergic
disorders at age 4 years: a whole population birth cohort study.
Pediatrics. 2001;108:E33.
22. Kovac K, Dodig S, Tjesić-Drinković D, Rao SM.
Correlation between asthma severity and serum IgE in asthmatic children
sensitized to Dermatophagoides pteronyssinus. Arch Med Res.
2007;38:99-105.
23. Mahesh PA, Kummeling I, Amrutha DH, Vedanthan PK.
Effect of area of residence on patterns of aeroallergen
sensitization in atopic patients. Am J Rhinol Allergy. 2010;24:e98-103.
24. Roberts G, Zhang H, Karmaus W, Raza A, Scott M,
Matthews S, et al. Trends in cutaneous sensitization in the first
18 years of life: results from the 1989 Isle of Wight birth cohort
study. Clin Exp Allergy. 2012;42:1501-9.
25. Ozol D, Koca C, Mete E, Yigitođlu R. Influence of
atopy on asthma severity in adult female patients. J Investig Allergol
Clin Immunol. 2008;18:36-40.
26. Moore WC, Bleecker ER, Curran-Everett D, Erzurum
SC, Ameredes BT, Bacharier L, et al. National Heart, Lung, Blood
Institute’s Severe Asthma Research Program. Characterization of the
severe asthma phenotype by the National Heart, Lung, and Blood
Institute’s Severe Asthma Research Program. J Allergy Clin Immunol.
2007;119:405-13.
27. Ponte EV, Souza-Machado A, Souza-Machado C,
Franco R, Cruz AA. Atopy is not associated with poor control of asthma.
J Asthma. 2012;49:1021-6.
28. Sinisgalli S, Collins MS, Schramm CM. Clinical
features cannot distinguish allergic from non-allergic asthma in
children. J Asthma. 2012;49:51-6.
29. Castro-Rodriguez JA, Ramirez AM, Toche P, Pavon
D, Perez MA, Girardi G, et al. Clinical, functional, and
epidemiological differences between atopic and non atopic asthmatic
children from a tertiary care hospital in a developing country. Ann
Allergy Asthma Immunol. 2007;98:239-44.
30. Hervás D, Milán JM, Garde J. Differences in
exhaled nitric oxide in atopic children. Allergol Immunopathol (Madr).
2008;36:331-5.
31. Travers J, Marsh S, Aldington S, Williams M,
Shirtcliffe P, Pritchard A, et al. Reference ranges for exhaled
nitric oxide derived from a random community survey of adults. Am J
Respir Crit Care Med. 2007;176:238-42.
32. Chng SY, Van Bever HP, Lian D, Lee SX, Xu XN,
Wang XS, et al. Relationship between exhaled nitric oxide and
atopy in Asian young adults. Respirology. 2005;10:40-5.
33. Mahut B, Peyrard S, Delclaux C. Exhaled nitric
oxide and clinical phenotypes of childhood asthma. Respir Res.
2011;12:65.
|
|
|
 |
|

