Table I shows the results of her
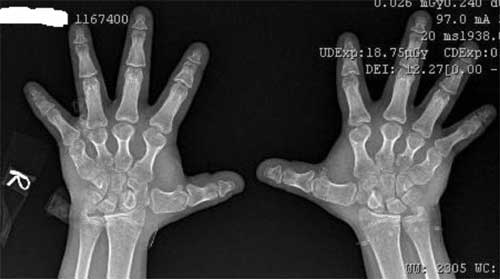
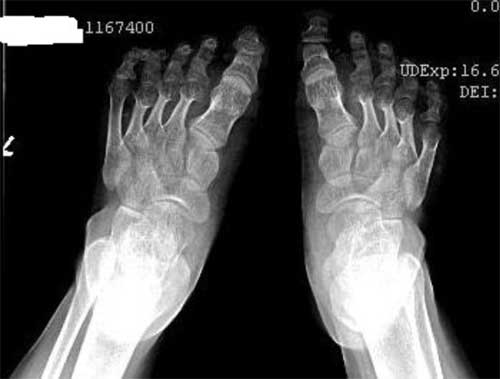
laboratory investigations. Radiograph of hands and feet showed
marked shortening of the metacarpals and metatarsals (Fig.
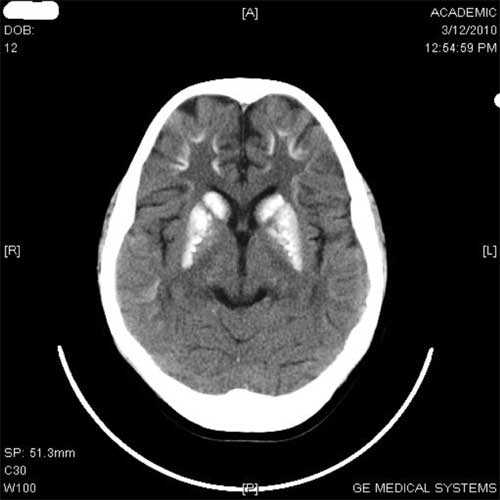
1 and 2). Neuroimaging of brain revealed intracranial
striopellidodentate calcification (Fig. 3).
Histopathologic examination of abdominal nodules revealed small
spicules to large masses of mature bone in the dermis suggestive of
primary subcutaneous calcification.
 |
|
Fig. 1 Brachydactyly of hands.
|
 |
|
Fig. 2 Brachydactyly of feet.
|
 |
|
Fig. 3 Bilateral striopeliodentate
calcification.
|
She was treated initially with intravenous (IV)
sodium valproate to control seizures along with IV calcium and
intramuscular vitamin D (Inj arachitol – 6 lac units). Later,
medications were changed to oral calcium (2 gdaily) and vitamin D (alphacalcidol
2 mcg daily). Associated hypothyroidism was treated with 50 mcg
levothyroxine daily.
Her mother also had abnormal morphological
features, round facies, short stature (height 145cm) and
brachydactyly of fingers and toes. Her serum calcium, phosphorus and
alkaline phosphatase levels were normal. It was reported that the
mother’s father was phenotypically similar to her. There is no
history of convulsions in the mother’s father. He died about ten
years before his granddaughter reported to us.
Two months after initial presentation, child was
readmitted with diabetes mellitus. Her serum C–Peptide levels were
low (0.3 ng/mL against a reference range of 1.1 – 5.0 ng/mL)
suggesting type 1 diabetes mellitus (T1DM). She was put on
subcutaneous insulin. Her blood sugar levels are well controlled on
this. On follow up for over 2 years, she has had no further seizures
after correction of her hypocalcemia.
Discussion
PHP manifests on account of genetic defects in
the hormone receptor adenylate cyclase system such that PTH does not
raise the level of calcium or lower the level of phosphorous. In
Type 1a PHP the defect is in the G protein which is a coupling
factor for PTH to activate c - AMP. The G-protein defect can impact
a large number of hormones besides PTH [1]. PHP Type Ia has
heterozygous loss-of-function in Gs-alpha unit and is inherited from
the mother. Patients are characteristically short statured with
stocky habitus, obesity, round facies, brachydactyly and soft-tissue
calcification. These features are typically known as the Albright
Hereditary Osteodystrophy (AHO) phenotype [1]. Striopallido-dentate
calcification, although uncommon, helps confirm the diagnosis of
Type 1a PHP [2]. PHP Type Ib does not have the AHO phenotype and
there is no resistance to other G-protein coupled hormones. PHP Type
II has normal phenotype. PPHP patients have features similar to Type
Ia PHP but without biochemical evidence of PTH resistance [3]. Our
patient has features of Type Ia PHP, including osteoma cutis and
striopellidodentate calcification. Her mother also had features
suggestive of PPHP.
Primary hypothyroidism and hypogonadism are the
associated hormone defects that occur most commonly. Hypothyroidism
may be apparent earlier in life, prior to development of
hypocalcemia of PHP. Reproductive dysfunction in the form of delayed
puberty, oligomenorrhea and infertility may also occur [4]. Our
patient had hypothyroidism. SMR was normal for age. She had not
attained menarche at 12 years of age.
Recent studies suggest that some of the actions
of insulin may be mediated by G protein [5-7]. This has been
demonstrated in animal models (Zucker rats) of both insulin
dependent and noninsulin dependent diabetes [8]. However, diabetes
has not been associated with PHP in humans previously. The child
reported here had low serum C-peptide level, suggestive of T1DM,
which is an unusual association. Type 2 diabetes has been reported
earlier with both PPHP and PHP [9,10]. Coincident hypovitaminosis D
in this child can result in low calcium and elevated PTH levels
similar to PHP. However the other phenotypical features of PHP
cannot be explained by vitamin D deficiency.
The child’s maternal grandfather was also short
"with features like the mother", suggesting he probably had either
PPHP or PHP (grandfather was not tested to determine if he had
hypocalcaemia). The sequence of inheritance in this pedigree adds
credibility to the suggestion that the disorder is influenced by
imprinting in a parent-of-origin dependent manner. The defective
gene is a dominant gene. However, if it is inherited from the father
the gene is only partially expressed (PPHP). If, on the other hand,
the gene is inherited from the mother it is fully expressed (PHP).
Even if the gene is only partially expressed, it has potential to be
fully expressed in the next generation depending on the sex of the
parent transmitting the gene. In this family study, the index case
acquired the disease (PHP) from her mother who had PPHP. She in turn
inherited PPHP from her father.
We did not test antibody for pancreas (autoantibodies
to islet cell cytoplasm (ICA), insulin (IAA), antibodies to glutamic
acid decarboxylase (GADA or GAD65) or ICA512 (IA2)). The results
would have helped to understand if the child’s diabetes was
associated with antibodies.
Contributors: All authors contributed to this
case report.
Funding: None; Competing interests:
None stated.
References
1. Lubell T, Garzon M, Anyane"Yeboa K, Shah B. A
novel mutation causing pseudohypoparathyroidism 1A with congenital
hypothyroidism and osteoma cutis. J Clin Res Pediatr Endocrinol.
2009;1:244-7.
2. Manabe Y, Araki M, Takeda K, Yokota S, Kimura
S. Pseudohypoparathyroidism with striopallidodentate
calcification—a case report and review of the literature.
3. Yamamoto M, Takuwa Y, Masuko S, Ogata E.
Effects of endogenous and exogenous parathyroid hormone on tubular
reabsorption of calcium in pseudohypopara-thyroidism. J Clin
Endocrinol Metab. 1988;66:618-25.
4. Marx SJ, Hershman JM, Aurbach GD. Thyroid
dysfunction in pseudohypoparathyroidism. J Clin Endocrinol Metab.
1971;33:822-8.
5. Rizzo MA, Romero G. The role of G proteins in
insulin signalling. J Basic Clin Physiol Pharmacol. 1998;9:167-95.
6. Malbon CC. Insulin signalling: putting the
‘G-’ in protein–protein interactions. Biochem J. 2004;380:e11-e12.
7. Robertson RP, Seaquist ER, Walseth TF. G
proteins and modulation of insulin secretion. Diabetes.1991;40:11-6.
8. Young P, Kirkham DM, Murphy GJ, Cowthorne CA.
Evaluation of inhibitory guanine nucleotide regulatory protein Gi
function in hepatocyte and liver membranes from obese Zucker (fa/fa)
rats and their lean (Fa/?) littermates. Diabetologia. 1991;34:564-9.
9. Wu CJ, Sheu WH. Type 2 diabetes in adults with
pseudopseudohypoparathyroidism. Diabetes Care. 1998;21:1575-6.
10. Kawakami A, Nagasaka S, Rokkaku K, Nakamura T,
Kusaka I, Yajima Y, et al. Pseudohypoparathyroidism, obesity,
and type 2 diabetes - A hypothesis. Diabetes Care. 1999;22:523.

