|
|
|
Indian Pediatr 2011;48: 969-971 |
 |
Azathioprine Hypersensitivity Presenting as
Sweet Syndrome in a Child with Ulcerative Colitis |
|
Mi Jin Kim, *Kee Taek Jang, and Yon Ho Choe
From the Departments of Pediatrics and *Pathology,
Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul,
Korea.
Correspondence to: Yon Ho Choe, Department of
Pediatrics, Samsung Medical Center, Sungkyunkwan University School of
Medicine, 50 Irwon-dong, Gangnam-gu, Seoul, 135-710, South Korea.
Email: [email protected]
Received: June 10, 2010;
Initial Review: July 01, 2010;
Accepted: August 05, 2010.
|
Sweet syndrome is a cutaneous lesion characterized by tender, red
inflammatory nodules or papules. We describe a pediatric case of Sweet
syndrome presenting 10 days after treatment with azathioprine. As
azathioprine is widely used in children with inflammatory bowel disease,
clinicians should be aware of this unusual adverse reaction.
Key words: Azathioprine, Children, Hypersensitivity, Sweet
syndrome.
|
|
Sweet syndrome, or acute febrile neutrophilic
dermatosis, is a cutaneous lesion characterized by tender, red
inflammatory nodules or papules, usually affecting the upper limbs,
face and neck. It can become generalized, and patients often are ill
with associated signs and symptoms, including malaise, high fever,
neutrophilia, elevated erythrocyte sedimentation rate and C-reactive
protein levels, which mimic an infectious process. It has rarely been
seen as a mani-festation of azathioprine hypersensitivity in adults
[1-4].
Case Report
A nine-year-old girl was referred for management
of refractory ulcerative Colitis (UC) that had been diagnosed one
year previously. She had no history of reported drug allergies and
had been prednisolone-dependent (2 mg/kg/day) for much of the
preceding year, with her disease flaring after attempts to reduce the
prednisolone dosage. During the hospitalization, she received
mesalazine treatment (48 mg/kg/day) without prednisolone, but it was
ineffective. She underwent azathioprine therapy (1 mg/kg/day) and 10
days later was hospitalized for fever (temperature of 39.2ºC), skin
rash and hematochezia.
Physical examination was significant for numerous
erythematous, painful, 1-3 mm vesicular lesions with central pustules
on her face and both arms (Fig. 1). Laboratory results
showed an elevated white blood cell count (13,470/µL) with
neutrophilia, microcytic hypochromic anemia (hemoglobin 9.2 g/dL, MCV
80.9 fl, MCH 24.8 pg), hyponatremia (132 mmol/L), and a markedly
raised erythrocyte sedimentation rate (89 mm/hr) and C-reactive
protein level (3.13mg/dL). Anti-nuclear antibody was negative, but
c-type anti-neutrophil cytoplasmic antibody (c-ANCA) was positive.
Her thiopurine methyltransferase (TPMT) activity was normal (18.2
U/ml RBC, reference range: 15.1-26.4 U/mL RBC). Blood cultures and
urinalysis were obtained and the patient was started on cefotaxime
(100 mg/kg/day) for a possible infectious etiology.
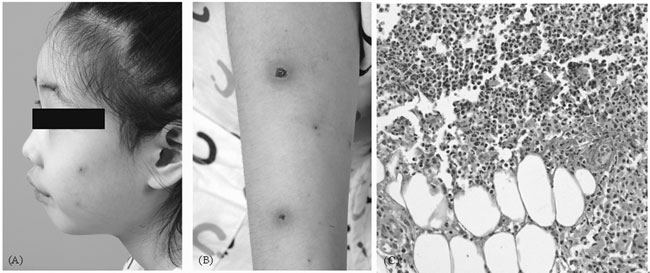 |
|
Fig. 1 Pustular and crust lesions
surrounded by erythema appeared on face (a) and arm (b) 10 days
after administration of azathioprine. (c) Skin biopsy of
pustular lesion shows massive neutrophilic infiltration in
entire dermis (H&E, x400). |
Two days after the cefotaxime treatment, the
patient still had a fever. The patient then received metronidazole
(30 mg/kg/day) for a week and prednisone (1.7 mg/kg/day). However,
she was febrile with shaking chills and nausea. Cultures taken from
blood and urine prior to antibiotic therapy were sterile. Biopsy
specimens and tissue cultures were taken from the pustular lesions.
Pathologic evaluation of skin biopsy showed massive neutrophilic
infiltrate in the entire dermis (Fig. 1). Tissue
culture results were negative for bacterial or fungal infection.
Based on the clinical course, a diagnosis of sweet syndrome was made.
As the patient’s fever had not subsided in spite of the
administration of antibiotics and steroid, we presumed that the sweet
syndrome was caused by the azathioprine and was not due to
inflammatory bowel disease. The azathioprine therapy was
discontinued. Within 48 hours, the patient’s fever abated and her
skin lesions improved. Following this improvement, the prednisolone
dose was reduced to 0.4 mg/kg per day without a recurrence of her
symptoms.
At follow-up after two weeks, there had been no
recurrences of her symptoms, and her UC was comparatively well
controlled by prednisolone and mesalazine treatment.
Discussion
The criteria for drug-induced SS have been
reviewed by many authors [3,5] and include abrupt onset of painful
erythematous plaques or nodules, histopathologic evidence of a dense
neutrophilic infiltrate without evidence of leukocytoclastic
vasculitis, pyrexia (temperature >38ºC), and a temporal relationship
between drug ingestion and clinical presentation, as well as
resolution after withdrawal. Our patient meets most of these
criteria.
ANCAs have been described in some cases, and may
be pathogenically relevant through the activation of neutrophils [6].
In our case, c-ANCA was positive. Kemmett, et al. [7] reported
the presence of c-ANCA in six of the seven patients with sweet
syndrome and speculated whether ANCA may be helpful in establishing
the diagnosis of sweet syndrome .
Azathioprine is a widely used immunosuppressive
agent that has been used increasingly as a steroid-sparing agent for
the treatment of Crohn’s disease and UC. Azathioprine rarely causes a
hypersensitivity syndrome which is characterized by fever, headache,
arthralgias, and rash, with possible cardiovascular, renal, lung, and
hepatic involvement [8]. Skin lesions include erythematous or
maculopapular eruptions, vesicules or pustules, urticaria, purpuric
lesions, erythema multiforme, or erythema nodosum. A case of acute
generalized exantematous pustules induced by azathioprine like our
case also has been reported [9]. Diagnosis is often missed or
delayed, as the clinical features are often misinterpreted as either
sepsis or an exacerbation of the underlying disease state. According
to previous studies [10], TPMT activity was not predictive of this
type of adverse effect.
The morphology of these skin lesions can mimic
that of several other mucocutaneous and systemic conditions. The
differential diagnosis includes infectious and inflammatory
disorders, neoplastic conditions, reactive erythemas, vasculitis.
Skin lesions and negative cultures help in the diagnosis. In
addition, negative test results for autoimmune diseases are important
for diagnosis. In our case, an infection focus or signs of an
autoimmune disease could not be detected. Clinical and
histopathologic findings supported the drug-induced sweet syndrome
and cessation of the drug caused a rapid regression in symptoms. In
patients without prior exposure to azathioprine, signs and symptoms
usually begin approximately two weeks from the initial azathioprine
exposure [1], which began after 10 days in this child.
We believe that azathioprine-induced sweet
syndrome may be under-diagnosed because it can easily be
misinterpreted as inflammatory bowel disease-related skin changes.
Contributors: All authors contributed to case
work-up and drafting the manuscript.
Funding: None.
Competing interests: None stated.
References
1. Garey KW, Streetman DS, Rainish MC.
Azathioprine hypersensitivity reaction in a patient with ulcerative
colitis. Ann Pharmacother. 1998;32:425-8.
2. Paoluzi OA, Crispino P, Amantea A, Pica R,
Iacopini F, Consolazio A, et al. Diffuse febrile dermatosis in
a patient with active ulcerative colitis under treatment with
steroids and azathioprine: a case of Sweet’s syndrome. Case report
and review of literature. Dig Liver Dis. 2004;36:361-6.
3. El-Azhary RA, Brunner KL, Gibson LE. Sweet
syndrome as a manifestation of azathioprine hypersensitivity. Mayo
Clin Proc. 2008;83:1026-30.
4. Yiasemides E, Thom G. Azathioprine
hypersensitivity presenting as a neutrophilic dermatosis in a man
with ulcerative colitis. Australas J Dermatol. 2009;50:48-51.
5. Su WP, Liu HN. Diagnostic criteria for Sweet’s
syndrome. Cutis. 1986;37:167-74.
6. Sarkany RP, Burrows NP, Grant JW, Pye RJ,
Norris PG. The pustular eruption of ulcerative colitis: a variant of
Sweet’s syndrome? Br J Dermatol. 1998;138:365-6.
7. Kemmett D, Harrison DJ, Hunter JA. Antibodies
to neutrophil cytoplasmic antigens: serologic marker for Sweet’s
syndrome. J Am Acad Dermatol. 1991;24: 967-9.
8. El-Azhary RA. Azathioprine: current status and
future considerations. Int J Dermatol. 2003;42:335-41.
9. Elston GE, Johnston GA, Mortimer NJ, Harman KE.
Acute generalized exanthematous pustulosis associated with
azathioprine hypersensitivity. Clin Exp Dermatol. 2007;32:52-3.
10. McGovern DP, Travis SP, Duley J,
Shobowale-Bakre el M, Dalton HR. Azathioprine intolerance in patients
with IBD may be imidazole-related and is independent of TPMT
activity. Gastroenterology. 2002;122:838-9.
|
|
|
 |
|

