|
|
|
Indian Pediatr 2010;47: 1059-1061 |
 |
Solitary Rectal Ulcer Syndrome: A Case Series |
|
N Suresh, R Ganesh and Malathi Sathiyasekaran
From Kanchi Kamakoti CHILDS Trust Hospital, Nungambakkam,
Chennai 600 034, India.
Correspondence to: Dr N Suresh, Senior Registrar in
Pediatrics, Kanchi Kamakoti CHILDS Trust Hospital, 12 A, Nageswara Road,
Nungambakkam Chennai 600 034, India.
Email:
[email protected]
Received: June 23, 2009;
Initial review: July 23, 2009;
Accepted: January 22, 2010.
Published online: 2010 March 15.
PII: S097475590900440- 2
|
|
Abstract
A retrospective analysis of the clinical profile,
endoscopic features and management of 22 children (age 18 months – 18
years) diagnosed as solitary rectal ulcer syndrome is presented. The
majority (81.8%) were ³8 years of
age. Rectal bleeding was the presenting feature in all the children.
Mucorrhea, constipation, tenesmus and rectal prolapse were observed
in 77.3%, 63.6%, 59% and 13.6% children, respectively. Colonoscopy
showed classical single rectal ulcer in 68.2% and multiple ulcers in
22.7%. Polypoidal and erosive lesions were documented in 4.5% each. The
medical management comprised of bowel training and high fibre diet for
all children. The other modalities included oral 5-amino salicylate
(59%), sucralfate enema (4.5%) and rectal mesalamine in 9%. 64% children
recovered and 13.6 % had recurrence of symptoms.
Key words: Colonoscopy, Lower gastrointestinal bleed, Rectal
ulcer.
|
|
S
olitary rectal ulcer syndrome (SRUS)
is an uncommon disorder of evacuation that affects all ages but is less
common in children compared to adults. SRUS is characterized by rectal
bleeding, mucorrhea, tenesmus, incomplete evacuation with characteristic
colonoscopic and histopathologic features(1). SRUS is occasionally
referred to as "the three- lies disease" since the lesion is not always
solitary or ulcerative or restricted to the rectum(2). The incidence of
SRUS in adults, is 1 in 1,00,000(3) whereas in children only few
case series have been reported. We report a series of 22 cases, probably
the largest ever reported from a pediatric tertiary referral center in
Chennai.
Methods
This is a retrospective study of children diagnosed as
SRUS based on colonoscopic findings and confirmed by histopathology during
the period May 2001-August 2009 in the Gastroenterology department of our
hospital. Case records of children who underwent colonoscopy during the
study period were reviewed. Only those children diagnosed as SRUS by
colonoscopy and confirmed by histopathology were included in the study.
The age, sex distribution, presenting symptoms, endoscopy features,
histopathology and treatment details were obtained from the records and
analyzed. Prior to colonoscopy all children undergo a detailed clinical
evaluation of all systems including rectal exami-nation and also
investigations, including complete blood count, stool examination for
parasites and ova and coagulation profile.
Results
During this eight-year period, 325 children less than
18 years underwent colonoscopy for various indications. Bleeding per
rectum was the most 6.7% (22 children), polyps in 27% and anal fissure in
15%. The age of children diagnosed as SRUS ranged from 18 months to 18
years (median age 10 years) of which 81.8% were
³8
years of age. The male to female ratio in this group was 1.4:1. Chronic,
intermittent rectal bleeding was the presentation in all the patients with
duration between 2 to 6 months in 60% and more than 6 months in 40%. Overt
rectal prolapse was present in 3 (13.6%), mucorrhea in 17 (77.3%),
straining during defecation in 14 (63.6%), digital evacuation in 6
(27.2%), constipation in 14 (63.6%) and abdominal pain/tenesmus in 13
(59%). None had evidence of liver disease or receiving medications that
may cause constipation.
Full length colonoscopy was done in all the 22 children
and single rectal ulcer was documented in 15/22 (68.2%) and multiple in 5
(22.7%). Ulcer size ranged from 0.2-3 cms and all were located with in 5
to 10 cms from the anal verge. In one child the lesion was polypoidal, and
erosions with surrounding erythema was seen in another child. One child
had, in addition, a rectal polyp situated 6 cm above the ulcer for whom
polypectomy was done and confirmed as juvenile polyp. All the children had
biopsy of the lesion and the histopathology revealed the diagnostic
hallmark of SRUS viz fibromuscular obliteration of the lamina
propria stroma with misorientation of smooth muscle cells (Fig.
1).
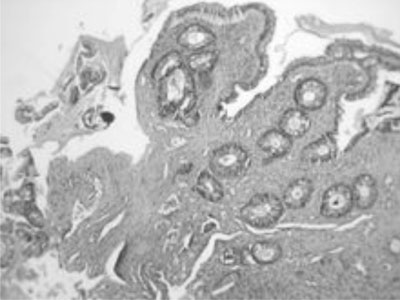 |
|
Fig. 1 Histopathology section showing
fibromuscular obliteration of the lamina propria (10 X). |
The medical management comprised of bowel training and
high fibre diet which was offered to all patients. 5-Amino Salicyalate (ASA)
was prescribed for 2 months in 13 (59%), topical applications such as
sucralfate enema in 1 (4.5%) and rectal mesalamine in 2 (9%). The youngest
child presented with overt rectal prolapse and frank bleeding per rectum.
This child was diagnosed earlier as probable cow’s milk allergy based on
the history and finding of nodular lymphoid hyperplasia on sigmoidoscopy
and was referred because symptoms did not subside with withdrawal of milk
proteins. Repeat endoscopy showed a large ulcer in the rectum which was
treated medically; however, there was recurrence of symptoms and worsening
of rectal prolapse. During the second admission, Meckel’s scan and
ultrasound of abdomen was done. The child required blood transfusion and
underwent surgery (rectopexy) for the rectal prolapse. The majority of
children improved symptomatically except three who came with recurrence of
bleed. The median follow up was 6 months (range 4-24 months). None of the
children had other specialized investigations such as anorectal manometry,
endoultrasound or defecography.
Discussion
SRUS is a benign rectal disorder of defecation which is
a well recognized entity in adults but often misdiagnosed or
under-diagnosed in children(4). The youngest child with SRUS reported so
far was 4.5 yrs(5); however, in this study, the youngest child was 1 year
and 6 months. The etiopathogenesis of SRUS is not well understood but is
probably secondary to ischemia and trauma to the rectal mucosa and
paradoxical contraction of pelvic floor(4). The excessive straining
generates a high intra-rectal pressure which pushes the anterior rectal
mucosa into the contracting puborectalis muscle resulting in pressure
necrosis of rectal mucosa. In addition, the anterior rectal mucosa is
frequently forced into the closed anal canal causing congestion, edema and
ulceration(6). Rectal bleeding was the most common presentation, observed
in all 22 cases. However, blood transfusion in SRUS is rare(7).
Colonoscopy in SRUS usually reveals rectal ulcer about 0.5 to 5 cm in
diameter on the anterior wall(2) and similar findings were also noted in
this study. Atypical features such as multiple ulcers in 30%, polypoidal
lesions in 25% or lesions situated beyond the rectum have been reported
and when present belies the term SRUS(2). Similar atypical features were
seen in this study including confirmed as SRUS on histopathology. Rectal
polyps have been reported as part of the spectrum of SRUS(8). In this
study one child had a juvenile polyp proved histopatho-logically in
addition to rectal ulcer, an observation that has not been reported in
literature so far. This spectrum of presentation in SRUS stresses the
importance of full length colonoscopy in children presenting with bleeding
per rectum. The differential diagnosis of SRUS includes inflammatory bowel
disease, inflammatory cloagenic polyps and ulcers related to topical
medications and infection, which can be differentiated by
histopathology(8). The characteristic histologic features of SRUS include
mucosal thickening with elongation and distortion of the glands, edema and
fibrosis of the lamina propria and extension of smooth muscle fibres
upward between the crypts(2,4). Rectal prolapse, either occult or overt is
well documented in SRUS varies from 15 to 59 %(9) and was a feature in
13.6% of this group.
Various therapeutic regimes have been tried and the one
universally recommended is high fibre diet and bowel training(2). Oral ASA
and topical agents such as steroids and mesalamine have not been found
effective(10). Sucralfate enemas and human fibrin sealant have shown
benefit in some patients(11). Argon plasma coagulation has been utilized
to treat disturbing hemorrhage(1). Behavioral modification or biofeedback
therapy improves both rectal blood flow and symptoms in more than 50% and
includes bowel habit training, avoiding excessive straining and
normalization of pelvic floor coordination. Surgery is indicated in those
with persistent bleeding per rectum not amenable to medical therapy and
includes rectopexy, excision of ulcer and rarely colostomy(1).
Contributors: NS and RG – Collected, analyzed the
data, reviewed literature and drafted the manuscript. MS revised the
manuscript for important content and will serve as guarantor
Funding: None.
Competing interests: None stated.
|
What This Study Adds?
• Solitary Rectal
Ulcer Syndrome should be considered in children presenting with
rectal bleeding, mucorrhea and excessive straining during
defecation.
|
References
1. Nagar AB, Proctor DD. Ulcers of the small and large
intestine. In: Feldman M, Friedman LS, Brandt LJ, Editors.
Sleisenger and Fordtrans. Gastrointestinal and Liver Disease: Pathophysiology,
Diagnosis and Management. Volume 2. 8th edition. Philadelphia: Saunders
Elsevier; 2006. p.2587-2598.
2. Perez LC, Vicent VM, Verge CR, Roman ALS, Milicua JM.
The three-lies disease: Solitary rectal ulcer syndrome. Rev Esp Enferm Dig
2007; 99: 663-666.
3. Dehghani SM, Haghighat M, Imanieh MH, Geramizadeh B.
Solitary rectal ulcer syndrome in children: a prospective study of cases
from southern Iran. Eur J Gastroenterol Hepatol 2008; 20: 93-95.
4. Esmaeily H. Histopathological misdiagnosis of
solitary rectal ulcer syndrome. Res J Biol Sci 2008; 3: 1171-1177.
5. Eigenmann PA, Lecoultre C, Cox J. Solitary rectal
ulcer: an unusual cause of rectal bleeding in children. Eur J Pediatr
1992; 151: 658-660.
6. Turck D, Michaud L. Lower gastrointestinal bleeding.
In: Walker WA, Goulet OJ, Kleinman RE, Sherman PM, Shneidear BL,
Sandarson IR, editors. Pediatric Gastrointestinal Disease: Pathophysiology,
Diagnosis and Management. Volume 1, 4th
edition. USA: BC Decker; 2004 .p. 266-280.
7. Ertem D, Acar Y, Karaa EK, Pehlivanoglu E. A rare
and often unrecognized cause of hematochezia and tenesmus in childhood:
Solitary rectal ulcer syndrome. Pediatrics 2002; 110: 279.
8. Al-Brahim N, Al-Awadhi N, Al-Enezi S, Alsurayei S,
Ahmad M. Solitary rectal ulcer syndrome: A clinicopathological study of 13
cases. Saudi J Gastroenterol 2009; 15: 188-192.
9. Gudbole P, Botterill I, Newell SJ, Sagar PM,
Stringer MD. Solitary rectal ulcer syndrome in children. J R Coll Surg
Edinb 2000; 45: 411-414.
10. Flet-Bersma RFJ, Cuesta MA. Rectal prolapse, rectal
intussusceptions, rectocele and solitary rectal ulcer syndrome.
Gastroenterol Clin North Am 2001; 30: 199-222.
11. Zargar SA, Khuroo MS, Mahajan R. Sucralfate
retension enema in solitary rectal ulcer. Dis Colon Rectum 1991; 34:
455-457.
|
|
|
 |
|

