|
Autoimmune hemolytic anemia
(AIHA) constitutes a group of diseases classified on the basis of
the temperatures at which the autoantibodies exhibit their maximal
reactivity to erythrocytes; warm AIHA has maximal reactivity at
37°C, and cold AIHA at 28-31°C [1]. Glucocorticoids and/or
intravenous immunoglobulins are the mainstay of treatment in the
majority of patients with warm AIHA [2,3]; however, when these
treatments fail patients often require cytotoxic drugs or
splenectomy.
We describe a 5-month old boy with a fulminant
type warm AIHA resistant to the standard treatment who was
successfully treated with rituximab.
Case Report
A 5-month-old boy with an uneventful prior
medical history was admitted to a regional hospital for
investigation following two days of sudden onset pallor, malaise and
anorexia. At presentation, his laboratory tests revealed a profound
anemia with a hemoglobin (Hb) of 2.6 g/dL, RBC 0.9×10 9/L,
MCV 98.2 fl, RTC 5.7%, WBC 15.4×109/L
and platelets 638×109/L;
total bilirubin levels were 127 micromol/L, with a direct fraction
of 19.2 micromol/L. Indirect and direct antiglobulin tests were
strongly positive, and nonspecific IgG autoantibodies were detected.
Serum immunoglobulin levels were within the normal range for his
age. Chest radiography was normal, and abdominal ultrasound revealed
a mild splenomegaly and hepatomegaly. He received intra-venous
immnunoglobulins and corticosteroids; however the hemolysis
continued and a transfusion of packed red cells was followed by
severe hemoglobinuria. He then underwent a partial exchange
transfusion with 450 mL of packed red cells to reduce the
circulating autoantibody levels, and was referred to our
Institution.
On admission, he was in a generally good
condition, with biochemical signs of hemolysis (LDH 1511 U/L, total
bilirubin 156 microml/L, with a direct fraction of 24.2 microml/L);
Hb 126g/l, RBC 3.6×10 9/L,
RTC 7.2% and hepatosplenomegaly. He was given further intravenous
corticosteroids (methylprednisolone 4mg/kg) and immunoglobulins
1g/kg for two days, however these again failed to halt the hemolysis
and the child was severely transfusion dependent; receiving two to
three transfusions of packed red cells per day. His general
condition significantly deteriorated (Fig. 1) with
increasing hepatosplenomegaly and rising bilirubin levels (total
bilirubin 764.7 micromol/L, with an increasing direct fraction of
637.9 micromol/L) and an LDH of 2780 U/L.
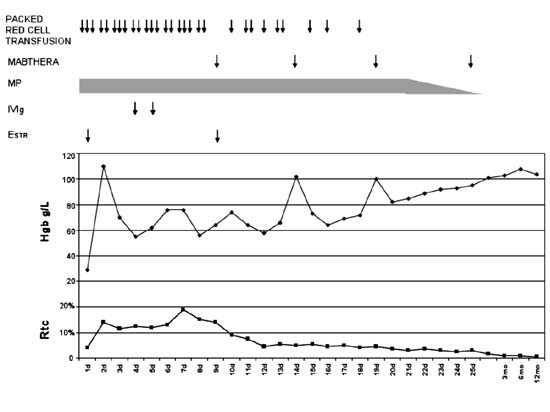 |
|
Fig. 1 Hemoglobin and reticulocyte
levels before and after the application of rituximab.
|
Eight days following admission, he was started on
rituximab 375mg/sqm weekly. After the second dose his transfusion
requirements and reticulocyte counts gradually reduced. The last
packed red cell transfusion was given two days after the third dose
of rituximab. The infant was given a total of four doses of
rituximab weekly, followed by monthly infusions of immunoglobulins
for the next six months and the hepatosplenomegaly gradually
regressed.
At 1 year follow-up, he was physically well
without any observed side-effects from the rituximab treatment.
Investigations revealed WBC of 10.9 × 109/L,
with normal lymphocyte counts for age, including absolute lymphocyte
counts (Ly 4690 cells/µl) and lymphocyte subsets analyzed through
flow cytometry on gated cells by direct immunophenotyping (CD
45+cells 40%, CD19+/CD45+ 27%, and CD20+/CD45+ 26%) at Becton
Dickinson, San Jose, USA. Immunoglobulin levels were also normal. He
remains well and free from hemolysis two years after treatment.
Discussion
Rituximab, intravenous immunoglobulins, immuno-suppressive
drugs and danazol have been shown to be effective in refractory AIHA
and in poor surgical candidates [1]. Rituximab is a humanized
monoclonal antibody IgG1/k directed against the CD20 antigen.
Although originally created for the treatment of non Hodgkin’s
lymphoma, the resultant depletion in normal B lymphocytes (which are
crucial for inducing and maintaining autoimmunity), has been found
to improve and/or cure a wide variety of autoimmune disorders [4,
5].
The mechanism behind this immunomodulatory effect
is not fully understood; as plasmocytes carry no CD20 expression on
their surface, and the direct destruction of antibody secreting
cells is not a plausible explanation. Furthermore, the autoantibody
titer do not correlate with clinical and laboratory improvement seen
following rituximab treatment. A possible mechanism of action lies
with the fact that B cells are antigen presenting cells, and as such
have a crucial role in activating and maintaining the response of
autoreactive T cells. The "immune decay" hypothesis postulates that
antibody-coated B cells are recognized by monocytes and macrophages,
which thus divert them away from interacting with the autoimmune
antibody complexes.
Our patient required immediate treatment with
packed red cell transfusions, immunoglobulins and corticosteroids
due to having an extremely low hemoglobin level, and thus
investigations for an underlying cause had to be postponed. However,
he had been a healthy child for the first 5 months of his life, with
a positive Coombs test, and had a good response to immunosuppressive
treatment with rituximab, thus congenital hemolytic anemia could be
excluded. Primary immunodeficiency was also excluded with the
presence of normal lymphocyte subsets and immunoglobulin levels, and
the regression of his hepatosplenomegaly during follow-up.
Our case demonstrated that as a single agent
rituximab was effective and safe in the context of a
life-threatening and fulminant warm antibody AIHA that had been
resistant to glucocorticoids. The efficacy of rituximab has also
previously been established in the treatment of children with
idiopathic AIHA, following organ transplant and during the course of
primary immunodeficiency [1, 3-6]. However, data on its use during
the first year of life is limited to information from small case
series and case reports [3,4,7]. One series described various
treatment durations with patients receiving between 4 and 35 courses
of rituximab [7]. Their patient whose treatment was initiated
earliest (11 days following diagnosis), required fewest rituximab
courses to achieve remission [7].
Our patient also achieved remission after 4 doses
of rituximab which was initiated 11 days after diagnosis, thus we
believe that an earlier introduction of treatment in case
of refractory AIHA in children offers the possibility of complete
remission with fewer cycles of rituximab. Our experience also
supports the belief that through the use of rituximab, the more
aggressive modes of treatment, such as splenectomy and the use of
cytotoxic drugs, could be avoided.
Acknowledgements: Dr Dushan Henry Atkinson
for his help in manuscript correction and interpretation.
Contributors: All contributors were involved
in manuscript preparation.
Funding: The study was partially supported by
the grant of the Serbian Ministry of Science and Education, grant
number 175056;
Competing interests: None stated.
References
1. Packman C. Hemolytic anemia due to warm
autoantibodies. Blood Rev. 2008;22:17-31.
2. Miloh T, Arnon R, Roman E, Hurlet A, Kerkar N,
Wistinghausen B. Autoimmune hemolytic anemia and idiopathic
thrombocytopenic purpura in pediatric solid organ transplant
recipients, report of five cases and review of the literature.
Pediatr Transplant. 2011;15:870-8.
3. Quartier P, Brethon B, Philippet P, Landman-Parker
J, Le Deist F, Fischer A. Treatment of childhood autoimmune
haemolytic anemia with rituximab. The Lancet. 2001;358:1511-3.
4. Zecca M, Nobili B, Ramenghi U, Perrotta S,
Amendola G, Rosito P, et al. Rituximab for the treatment of
autoimmune refractory hemolytic anemia in children. Blood.
2003;101:3857-61.
5. Giulino L, Bussel J, Neufeld E, Pediatric and
Platelet Immunology Committees of the TMH Clinical Trial Network.
Treatment with rituximab in benign and malignant hematologic
disorders in children. J Pediatr. 2007;150:338-44.
6. Kim J, Thrasher J, Jones A, Davies EG, Cale
CM. Rituximab for the treatment of autoimmune cytopenias in children
with immune deficiency. Br J Haematol. 2007;138:94-6.
7. Svahn J, Fioredda F, Calvillo M, Molinari AC,
Micalizzi C, Banov L, et al. Rituximab-based
immunosuppression for autoimmune haemolytic anaemia in infants. Br J
Haematol. 2009;145:96-100.
|

