|
|
|
Indian Pediatr 2009;46: 681-698 |
 |
Guidelines
for Diagnosis and Management of Childhood Epilepsy |
|
Expert Committee on Pediatric Epilepsy, Indian Academy of Pediatrics
Correspondence to: Dr Neeta Naik, Co-convenor, Expert
Committee on Pediatric Epilepsy, IAP; Epilepsy Research Centre for
Children, 111, Sundaram, First Floor, Opposite Cinemax, Sion East, Mumbai
400 022, India.
E-mail: [email protected]
Manuscript received: November 20, 2007;
Initial review: November 29, 2007;
Accepted: April 28, 2009.
|
|
Abstract
Justification: Seizures constitute the most
common neurological problem in children and the majority of epilepsy has
its onset in childhood. Appropriate diagnosis and management of
childhood epilepsy is essential to improve quality of life in these
children. Evidence-based clinical practice guidelines, modified to the
Indian setting by a panel of experts, are not available.
Process: The Indian Academy of Pediatrics
organized a consensus meeting on the diagnosis and management of
childhood epilepsy on 22-23 of July 2006 at Mumbai. An expert committee
was formed consisting of pediatricians, pediatric epileptologists,
pediatric and adult neurologists, electrophysiologists,
neuroradiologists, neurosurgeons and intensivists. A consensus was
reached during the discussion that followed presentation by each of
these experts. The process of updating these recommendations and
arriving at consensus continued till 2009.
Objectives: To develop practice guidelines for
diagnosis and management of childhood epilepsy.
Recommendations: Recommendations for diagnosis
and management of following childhood seizures and epilepsies are given:
neonatal seizures, acute symptomatic seizures, neurocysticercosis,
febrile seizures, idiopathic partial and generalized epilepsies, first
unprovoked seizure, newly diagnosed epilepsy, catastrophic epilepsies of
infancy, refractory epilepsies of older children and adolescents,
epilepsy with cognitive deterioration and status epilepticus.
Key words: Childhood Epilepsy, Diagnosis, India, Management,
Refractory epilepsy.
|
|
S
eizures constitute the commonest
neurological problem in children with significant epilepsy having its
onset in childhood. A considerable treatment gap exists in developing
countries due to poverty, stigmatization, and lack of trained manpower(1).
Evidence-based clinical practice guidelines can improve the quality of
care(2).
An expert consensus meeting was held at the PD Hinduja
National Hospital, Mumbai on July 22-23rd 2006 under the IAP 2006 action
plan. The aim was to produce a practice parameter for diagnosis and
management of epilepsy in the Indian context. All 22 experts (Annexure
I) had several years of experience and publications in
epilepsy. Epilepsy subtopics were assigned to each expert with a format of
five common questions faced by a practicing pediatrician. A manuscript and
presentation were prepared by each expert, using evidence from the medical
literature. Emphasis was placed on the resource-poor Indian context, which
often makes guidelines from developed countries difficult to apply. The
consensus statement was prepared and updated on a continuous basis till
2009.
Objectives
To provide easy, quick and practical guidelines for
diagnosis and management of acute symptomatic seizures; and newly
diagnosed and refractory childhood epilepsy to the practicing
pediatrician.
Recommendations
1. Neonatal Seizures
Neonatal seizures are often acute symptomatic due to
underlying brain insults. Focal clonic, multifocal clonic, and focal tonic
seizures are usually accompanied by ictal EEG activity while subtle,
generalized tonic and myoclonic episodes may be non-epileptic as they are
not associated with electrographic ictal activity(3). True seizures are
often accompanied by open eyes(4). Non-epileptic phenomena like
jitteriness and benign sleep myoclonus should be differentiated. Serum
glucose, electrolytes, calcium and magesium must be done in all(5,6). CSF
studies and culture must be done in all except when the diagnosis is
definite e.g. hypoxic ischemic encephalopathy. A portable 60 minute EEG by
a trained technician and interpreter is useful in recognizing subclinical
seizures, epileptic encephalopathies and prognosis(6). A cranial
ultrasound is the minimum imaging required, but an in-house MRI with
diffusion tensor imaging is the modality of choice, done immediately for
aetiology and at 3-6 months for prognosis(6). Management should be done as
per Fig.1(5-7).
 |
|
Fig 1. Algorithm for
management of neonatal seizure. |
Oral phenobarbitone should be continued till discharge
or up to 3 months (especially in those with an abnormal neurologic
examination).
2. Acute Symptomatic Seizures
A seizure occurring within a week of an acute brain
insult (trauma, infection, toxic, metabolic or vascular insult) is called
an acute symptomatic seizure(8). Future risk of unprovoked seizures is
only 3-10%.
Serum calcium, magnesium, electrolytes and glucose
should be estimated for all children. Lumbar puncture should be done in
febrile infants and in those with suspected meningoencephalitis. A plain
CT scan is indicated in traumatic brain injury and a contrast enhanced CT
scan is indicated in children above 2 years, especially those presenting
with convulsive seizures, focal seizures, cluster of seizure (9), or focal
neurological deficits to rule out granuloma.
In a hypocalcemic breastfed infant, an underlying
vitamin D deficiency state in the child and the feeding mothers should be
corrected(10). Anti-epileptic drugs (AED) are required in the acute phase
and can be withdrawn in a week in acute traumatic brain injury(11) or in 3
months in illnesses with parenchymal involvement (e.g. CNS
tuberculosis or pyogenic meningitis with parenchymal involvement).
3. Febrile Seizures
A simple febrile seizure occurs between the age of 6
months to 5 years. Complex febrile seizures are characterized by partial
onset, duration ³15
minutes, or multiple episodes in the same illness(12). Late onset febrile
seizures, persistent febrile seizures, generalized epilepsy and febrile
seizure plus (GEFS+) and febrile status epilepticus (FSE) are part of the
spectrum of febrile seizures(13).
Lumbar puncture should be done in children with a
suspected meningitis, especially in infants(13). EEG and neuroimaging have
no role in simple febrile seizures(12).
Management includes definitive diagnosis, restraint in
investigations, treatment of an acute episode, prophylaxis for future
episodes and family counseling(12). Role of defervescence in preventing
febrile seizures is questionable(13). Parents can be taught to use rectal
liquid diazepam (0.5 mg/kg) or buccal or nasal(12,15) midazolam (0.3
mg/kg) for acute termination of seizures that last for two minutes or
more.
Any prophylaxis of febrile seizures reduces the
recurrence of seizures but does not reduce the risk of future epilepsy.
Intermittent prophylaxis with oral clobazam in a dose of 0.75 mg/kg for
2-3 days in 2 divided doses during fever is useful to prevent recurrence.
Febrile status, complex and recurrent febrile seizures (>6/year in spite
of intermittent prophylaxis) may need EEG, neuroimaging and continuous
prophylaxis with AED. Phenobarbitone and valproate may be used in infants
and older children respectively, for 1-2 years(12). Carbama-zepine and
phenytoin are not useful.
4. Granuloma in Children
New-onset partial or generalized convulsive seizures
occurring in clusters in an otherwise normal child is the commonest
presentation of single small contrast enhancing CT lesion (SSECTL);
necessitating neuroimaging, except when an idiopathic epilepsy syndrome is
established with EEG(16). The commonest etiology is neurocysticercosis (NCC)
followed by tuberculomas. Parenchymal NCC cysts should be classified as an
active vesicular form (cystic, without enhancement or edema), a
transitional colloidal/granular-nodular form (ring/disc enhancement with
edema) or an inactive form (non-enhancing calcified lesions without
edema). Active lesions when accompanied by edema often produce a focal
background asymmetry on the EEG(17).
NCC vs. tuberculoma: On neuroimagings the
presence of an eccentric scolex in a cystic lesion is pathognomonic of a
NCC. Large (>2cm), often multiple, isodense lesions with shaggy borders
and location in the posterior fossa are likely to be tuberculomas(18). MR
Spectroscopy may help differentiate the two(19).
Cysticidal treatment is beneficial and recommended
strongly in live NCC cysts and transitional NCC granulomas(20).
Albendazole for a period of 7(20,21) or 28(20) days in a dose of 15 mg/kg
in 2 divided doses is the treatment of choice. Prednisone should be used
at 1mg/kg/day, 3 days before starting albendazole and continued for a
total of 7 days to reduce the risk of cerebral edema at the time of cyst
breakdown. A fundal examination should be performed before use of
cysticidal drugs as ophthalmic lesions are an absolute contraindication
for medical therapy.
AEDs are given in acute symptomatic seizures due to
active lesions (cystic lesions, granulomas) till such time that they
disappear or become inactive (no edema, no enhancement, calcified) –
usually for a period of 6 months(22). Inactive calcified lesions
presenting with seizures either de novo or as relapses should be
considered remote symptomatic and should be treated till a 2 year seizure
free period is achieved. Repeat imaging should be done to check the
resolution of lesion after 6 month, if the child is clinically well or
earlier, if the initial diagnosis was insecure or if the child is
symptomatic.
5. Idiopathic Partial Epilepsies in Childhood
Benign epilepsy with cento-temporal spikes
(Benign rolandic epilepsy, BECT)
This should be considered when a normal school aged
child presents with brief and infrequent, partial, nocturnal, hemi-facial,
sensory or motor seizures. An awake-cum- sleep EEG is necessary, as it
displays a characteristic pattern of sleep activated runs of centro-temporal
spikes or sharp waves. The syndrome has an excellent prognosis with
remission in most cases by the age of 15-16 years(23, 24).
Panayiotopoulos syndrome (Early-onset childhood
epilepsy with occipital paroxysms, CEOPs)
CEOP should be considered when a normal preschool (3-5
yrs) child presents with severe nocturnal vomiting, followed by eye
deviation and status epilepticus, usually hemiclonic. This syndrome has an
excellent outcome(25). A later-age Gastaut variant of CEOPS is usually
characterized by diurnal simple partial seizures with visual
hallucinations or amaurosis and migraine like headaches. This has a
variable prognosis and can persist into adult life. Neuroimaging is
considered in cases with an abnormal perinatal history or examination,
atypical EEG, or poorly controlled seizures.
Treatment with AED is not required when seizures are
infrequent, but parental counseling is a must. In long term therapy,
carbamazepine or valproate are preferred. The syndrome may evolve
atypically with frequent refractory seizures, scholastic deterioration
and/or behavioral changes; more often with the use of carbamazepine,
emphasizing the need of clinical monitoring (26).
6. Idiopathic Generalized Epilepsies
Childhood absence epilepsy (Petit-mal, CAE)
This should be suspected in a normal school age child
with frequent absence seizures often upto a hundred a day. These occur in
the awake state with sudden staring, unresponsiveness and minor brief
automatisms, leading to interruption of ongoing activity and unassociated
with any post ictal abnormality(23). GTC seizures are unusual. Pre-cipitation
of seizure by hyperventilation is a simple clinical diagnostic test.
Atypical absence seizures are prolonged, seen usually in catastrophic
pediatric syndromes with neurocognitive deterioration.
An EEG showing a typical pattern characterized by
frontally predominant generalized bursts of 3 Hz spike wave complexes with
abrupt onset is diagno-stic. There is no role for routine neuroimaging.
Valproate and ethosuxsimide (presently unavailable)
followed by lamotrigine(27) and the benzodiazepines are the drugs of
choice. Response to AEDs should be confirmed by repeat EEG with
hyperventilation to check the disappearance of typical EEG pattern.
Treatment is for a minimum seizure free period of 2 years with a normal
EEG at discontinuation.
Idiopathic generalized epilepsies of adolescent
When a child presents with absence, myoclonic or
generalized tonic, clonic seizures for the first time after 10 years, a
diagnosis of idiopathic generalized epilepsies of adolescent onset is
considered. EEG shows generalized paroxysms of spike or polyspikes wave
discharges. Photosensitivity is common. Juvenile myoclonic epilepsy (JME),
juvenile absence epilepsy (JAE) and epilepsies with only GTC seizures
should also be considered in diagnosis(28).
JME presents in adolescents with history of early
morning predominant upper limb myoclonic jerks leading to the patient
dropping objects. This occurs often in sleep-deprived individuals,
especially if suddenly awakened. JAE is similar to CAE, though the numbers
of absences are much less and the onset is usually later. GTC seizures
typically occur on awakening or in the evening(23).
Sodium valproate is the most effective drug in most
cases of idiopathic generalized epilepsies(29), but it may cause weight
gain, hair loss, and menstrual irregularities and has a higher incidence
of fetal teratogenicity. Therefore, lamotrigine may be preferable in
adolescent girls. Due to its lower cost, phenobarbitone may be used in
poor patients with resource constrains, with only generalized tonic clonic
seizures, but will not control myoclonic or absence seizures.
Carbamazepine and phenytoin may worsen the syndromes. Lifestyle
adjustments include avoiding precipitating factors like sleep deprivation
and alcohol consumption.
Seizure control is achieved in 80% with monotherapy.
Withdrawal of AEDs results in relapse in more than 90%, especially in JME
and JAE, and often need low dose lifetime medication. In cases of
epilepsies with only generalized tonic clonic seizures, a single trial of
AED withdrawal after a 2 years of seizure free interval may be tried, but
risk of recurrence is high and lifetime AED may be needed.
7. Investigations for Epilepsy
Electroencephalography (EEG)
When should an EEG be done?
EEG is recommended as a part of initial evaluation in
all children presenting with an episodic event. Epileptiform abnormalities
support a clinical diagnosis of seizure, help in the diagnosis of specific
syndromes and predict seizure recurrence(30); how-ever, a normal EEG does
not rule out epilepsy. The EEG interpretation is reliable only when it is
well recorded and interpreted by an experienced EEG reader with at least
one year of training in the same.
In the child with uncontrolled epilepsy, a repeat EEG
helps in reclassifying the syndrome. Before AED discontinuation, an EEG
aids in predicting the risk of recurrence in most syndromes barring a few
e.g. BECTS.
In children with unexplained cognitive, neurobehavioral
or scholastic deterioration; an EEG may help in diagnosis of specific
disorders like SSPE, or epileptic encephalopathies like electrical status
in slow wave sleep (ESES), and non-convulsive status epilepticus. There is
no place for routine follow-up EEGs in patients who are doing well.
How should an EEG be done(31)?
• EEG should be recorded 3-4 days after the last
seizure to avoid post-ictal slowing from interfering with the
interpretation.
• A sleep EEG after deprivation should be part of all
routine recordings in children above the age of three years.
• Minimum activation procedures like hyperventilation
and photic stimulation should be used.
• Omission of AED prior to EEG recording is not
recommended.
• Simultaneous video-EEG is useful in differentiating
non-epileptic events from true seizures and for pre-surgical evaluation.
EEG in status epilepticus(SE):
A portable EEG can be done in children with convulsive
SE who do not regain consciousness as expected, so as to exclude an
ongoing nonconvulsive status epilepticus (NCSE). Continuous EEG
moni-toring is desirable in refractory SE when pentothal or propofol are
being used for dose titration.
Neuroimaging
MRI is more sensitive than CT and is the modality of
choice. CT retains a role in detecting calcification and in acute
situations like head trauma, status epilepticus, and epilepsy, where
granulomas are a possibility. MRI protocol should be adapted to the age of
the child and the type of epilepsy syndrome(32). Neuroimaging is not
recommended in benign epilepsies. High resolution MRI with special
techniques is recommended for delineating the epileptogenic zone and the
eloquent cortex in pre-surgical evaluation. The preferred sequences are
T1W (preferably, inversion recovery), T2W and PD fast spin echo,
Fluid-Attenuated Inversion Recovery (FLAIR), 3D T1 acquisitions with 1-2
mm partitions (better anatomy and morphometry).
8. Management of First Unprovoked Seizure
A good history is most important for diagnosis of a
seizure. Open eyes, eye and head deviation, incontinence, tongue-bite are
fairly specific for a seizure, whereas unresponsiveness, confusion, clonic/tonic
movements are suggestive, though these may be prominent in non-epileptic
events as well(33). If the child is less than 6 months, admission for
observation and evaluation is recommended.
• EEG preferably 3-4 days after the seizure is
recommended in all cases(34).
• Neuroimaging would be needed when there are seizure
cluster, focal deficits, altered sensorium, focal EEG background change,
etc(34).
• In the first seizure, AED should not be
recommended, but a detailed discussion with the parents is necessary.
Exceptions are status epilepticus due to high rate of recurrence(35) or
severe parental anxiety. Home management of seizures includes use of
rectal diazepam/buccal or nasal midazolam(36) in seizures lasting for
more than 2 minutes.
9. Management of Newly Diagnosed Epilepsy
• Long term AED treatment should be started after
second seizure(37). The aim of treatment is complete seizure control
without significant adverse effects. AED is based on the predominant
seizure type or syndrome type with possible adverse effects and
co-morbidities taken into account(37,38).
• All drugs are started in low doses and increased
gradually upto a maximum dose till seizure control is achieved or side
effects appear.
• Dosage needs to be adjusted to the child’s daily
activity. Extended release formulations in twice a day dosing are
preferable(39).
• If no control is obtained with maximum doses of the
first drug, then a second first line drug is initiated and the first
drug tapered(38). If partial control is achieved(37), then a second AED
should be added. All efforts should be made to use only rational
polytherapy.
• There are no significant differences in the
efficacy or tolerability of the four major first line anticonvulsants (phenobarbitone,
phenytoin, valproate and carbamazepine) and any one can be used
first(40), based on side effect profile. Carbamazepine and valproate
appear to be better tolerated than phenobarbitone and phenytoin.
• A seizure diary should be kept by the parents.
• Therapeutic drug monitoring is useful in only few
situations, including breakthrough or refractory seizures, to assess
compliance, for diagnosis of clinical toxicity or with use of phenytoin,
which has dose dependent pharmacokinetics(41).
• In most epilepsy, AED is withdrawn after 2 year of
seizure freedom. Adolescent onset, remote symptomatic epilepsy and
abnormal EEG after 2 years are predictors of relapse(42), warranting
drug withdrawal after 4 years(43). Drug withdrawal is over 3-6
months(44,45) and one drug at a time in cases of polytherapy.
10. Conventional Antiepileptic Drugs
Phenobarbitone
Phenobarbitone could be used as a first line AED in
neonatal seizures(46), in the first two years of life for partial/GTC
seizures(47) and in neonatal and early infantile status epilepticus(SE).
The dosage varies between 3-6 mg/kg/day given as a single night-time dose
for routine use and 20 mg/kg given as loading for SE. Since deleterious
cognitive and behavioral side effects remain a concern, it should be
avoided in schoolgoing children.
Phenytoin
Though effective, should not be preferred as a primary
AED in newly diagnosed epilepsy, especially in infancy, as levels
fluctuate frequently in infants, making monitoring of drug levels
imperative(41), and in adolescent girls as cosmetic side effects may be
unacceptable(48). Maintenance dosages in older children are between 5-6
mg/kg given in one or two divided doses, but infants may need upto 15-18
mg/ kg in 3-4 divided doses.
Valproate
As a result of its broad spectrum of efficacy,
valproate could be the drug of choice for most children with newly
diagnosed epilepsy, like idiopathic generalized epilepsy (CAE, JAE, BMEI,
and JME), epilepsies with prominent myoclonic seizures or with multiple
seizure types, and photosensitive epilepsies(49). However, in adole-scent
girls or obese patients, one may not use it as first line agent due to
concerns of weight gain, hair loss and aggravation of polycystic ovarian
disease (PCOD), which should be specifically looked for(50). Hair loss may
be reduced by use of supplemental biotin(51). It could be used in partial
epilepsies in infants where carbamazepine might precipitate generalized
seizures and in refractory status epilepticus. The dose averages between
10-40 mg/kg/day. Twice-a-day dosing is preferred with extended release
preparations(39), except in syrup (3 times a day). Parents should be
counseled regarding danger symptoms and signs of hepatitis, like nausea,
vomiting, drowsiness etc, especially in children below the age of 2 years,
those on polytherapy and those with associated IEM, necessitating routine
monitoring of LFT. Enzyme elevation upto twice normal or borderline
elevation of ammonia can be disregarded when asymptomatic. The drug must
be stopped immediately in all symptomatic patients irrespective of enzyme
levels. In case the cause of the hepatitis becomes clear e.g.
hepatitis A confirmed by serology, then valproate could be restarted after
the hepatitis has resolved. In cryptogenic hepatitis it is best avoided.
Carnitine supplements are not routinely recommended(52).
Carbamazepine
It is the drug of first choice for all newly diagnosed
partial epilepsies(53), after the age of 2 years. The dose varies between
10-30 mg/kg in the form of twice a day dosing and preferably given as slow
release preparations(54,55), if syrups are used they should be given three
times a day. Carbamazepine may induce or exacerbate generalized seizures
like infantile spasms, myoclonic, tonic and absence seizures in the
younger child(56). Paradoxically, it may exacerbate partial seizures as
well, in benign partial epilepsies. It may worsen the EEG with
deterioration in cognition, behavior and language & can rarely precipitate
electrical status in slow wave sleep (ESES)(57). Parents should be
informed about the common side effects like appearance of new seizures,
deterioration of school performance or appearance of rash (Steven’s)
Johnson’s and Drug Rash Eosinophilia and Systemic Symptoms- DRESS syndrome
shared with phenytoin and pheno-barbitone) which should be reported
immediately. A routine hematological monitoring is not recommended.
11. Newer Antiepileptic Drugs
Table I summarizes the guidelines for new
antiepileptic drugs. Table II depicts the dosage and
side-effects of common antiepileptic drugs.
TABLE I
Guideline For New Drugs In New Onset And Refractory Epilepsy
| |
Clobazam |
Lamotrigine |
Levateracetam |
Topiramate |
Oxcarbazepine |
Tiagabine |
| New Onset |
No |
Yes (JME, CAE) |
No |
No |
Yes (Partial ) |
No |
| Partial |
|
Yes |
|
|
|
Yes |
| Absence |
|
Yes |
|
|
|
No |
| Myoclonic |
|
Yes |
|
|
|
No |
| GTC |
|
Yes |
|
|
|
No |
| Refractory |
|
|
|
|
|
|
| Partial |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| Absence |
Yes |
Yes |
Yes |
Yes |
No |
No |
| Myoclonic |
Yes |
Yes |
Yes |
Yes |
No |
No |
| Spasm |
No |
Yes |
No |
Yes |
No |
No |
| LGS |
Yes |
Yes |
Possible |
Yes |
No |
No |
TABLE II
Doses And Side Effects Of Common Antiepileptic Drugs
| Drugs |
Daily dose |
Common side effects |
| Phenobarbitone |
3-8 mg/kg |
Hyperactivity, academic deterioration, reversal of
sleep cycles |
| Phenytoin |
5-15 mg/kg |
Poor seizure control due to fluctuating drug levels,
cosmetic side effects, hirsutism, ataxia |
| Valparin |
10-60 mg/kg |
Nausea, vomiting, loss of appetite,
weight gain, irregular menstruation, alopecia, somnolence |
| Carbamazepine |
10-30 mg/kg |
Drug rash, worsening seizures, rarely worsening
school performance |
| Oxcarbazepine |
20-45 mg/kg |
Somnolence, vomiting (hyponatremia), seizure
exacerbation |
| Lamotrigine |
0.2- 15 mg/kg |
Drug rash, Steven-Johnson syndrome |
| Clobazam |
0.4-1.2 mg/kg |
Behaviour changes, aggression, sleep disturbances,
constipation, weight gain |
| Topiramate |
3-9 mg/kg |
Cognitive/language deterioration, fever, acidosis in
infancy |
| Levateracetam |
15-45 mg/kg |
Behaviour changes |
| Tiagabine |
0.5-2 mg/kg |
Somnolence, Seizure exacerbation |
Clobazam
It can be used as intermittent therapy(57,58) or as
continuous add-on drug(59-61); is not recommended as monotherapy for newly
diagnosed epilepsy.
• Intermittent use
(a) Febrile seizure prophylaxis to prevent
acute seizure recurrence(57,58).
(b) Reflex epilepsy e.g. hot water epilepsy
(just before a hot water bath).
(c) Catamenial epilepsy (Straddling the
menstrual period).
(d) During seizure clusters.
• Add-on therapy
(a) Refractory partial(59) and generalized
epilepsies(60,61).
(b) Certain epileptic syndromes like LGS,
Myoclonic-astatic epilepsy (Doose’s syndrome), Dravet’s syndrome,
Continuous spike and wave in slow wave sleep (CSWS).
The starting dose is 0.1-0.25 mg/kg, which is titrated
against seizure control every seven days till 0.75 mg/kg- 1mg/kg is
reached. It should be given in two divided doses or as a single nightly
dose; it should be withdrawn very gradually to avoid withdrawal seizures.
Tolerance may not be a significant problem(62).
Common adverse events to be recognized include
behaviour changes (aggression, hyperactivity), sleep disturbances,
constipation and weight gain.
Oxcarbazepine
Can be used as monotherapy in newly diagnosed partial
epilepsy for children above 4 years of age(63), if affordable and
available. It can be used for add-on therapy in refractory partial and
secondary generalized epilepsy(64,65). Start with a dose of 10 mg/kg;
titrated upwards weekly, guided by seizure control, to a maximum of 40
mg/kg. Though once daily preparations are marketed, it is prudent to give
this drug in two divided doses. An abrupt switchover from CBZ to OXZ can
be done in a dose ratio of 2:3(66). No routine monitoring of drug levels,
blood counts or sodium is recommended, unless symptomatic (vomiting,
drowsiness or increased seizures).
Lamotrigine
Monotherapy in newly diagnosed generalized epilepsy
(absence and myoclonic)(67) and in other partial(68)/generalized
epilepsies, and in specific epilepsy syndromes like idiopathic generalized
epilepsy in teenage years, especially girls (as first choice)(29).
Occasionally, myoclonic jerks maybe paradoxically worsened by lamotrigine,
especially in JME.
Add-on in refractory generalized epilepsies like
absence, tonic and tonic-clonic and syndromes like LGS and in refractory
partial epilepsies also(64).
The dose should initially be 0.5 mg/kg (alone), 0.2
mg/kg (with VPA), and 0.6 mg/kg (with phenobarbitone, phenytoin,
carbamazepine); it should be doubled every 2 weeks to a maximum of 15mg/kg
(alone) and 5mg/kg/day (with VPA) and higher when used with enzyme
inducers. LTG has to be titrated slowly to prevent rashes and Stevens
Johnson syndrome.
Topiramate
It can be used as a second line add-on agent in
refractory partial and generalized epilepsies as well as Lennox Gastaut
syndrome(64). It maybe particularly useful in certain syndromes like
infantile spasms(69,70) and Dravet’s syndrome(71). At present, its use as
first line monotherapy in newly diagnosed epilepsy is not recommended
because of a significant adverse effect profile.
It should be started at a dose of 0.5-1 mg/kg in bid
doses, escalated weekly or biweekly, upto maximum of 5-10mg/kg (72);
Higher doses (10-30 mg/kg) and rapid escalation (every 3 days) are
considered in special situations (infantile spasms, status epilepticus);
however, there could be a higher incidence of adverse events with high
doses.
Clinical monitoring for adverse effects like weight
loss, eye symptoms like blurring, redness, watering and eye pain
(glaucoma/myopia), metabolic acidosis(73) and oligohydrosis(74) is
necessary in all cases. Decreased appetite and weight loss are expected
and should be communicated to the caregivers. Cognitive adverse effects
can be minimized by converting to topiramate monotherapy, if possible.
Hydration should be maintained and calcium supplements
should be avoided to minimize risk of renal stones.
Levatiracetam
It should be used only as an add-on drug to refractory
partial(75,76) and some generalized epilepsies like, refractory absence or
progressive myoclonic epilepsies(77). It is not recommended to be used as
a first-line agent in newly diagnosed epilepsies, though recent data
support a role in the idiopathic generalized epilepsies of adolescents (JME
etc). Behavioral adverse effects like aggression are the most common
adverse effects, rarely a paradoxical increase in seizure frequency may
occur and this should be monitored carefully.
The usual effective dose is between 20-60 mg kg /day.
One can start at 20 mg/kg in two doses and increase every 1-2 weeks till
60 mg/kg/day.
Tiagabine
As add-on in refractory partial seizures(78). It is not
recommended as monotherapy in children with newly diagnosed epilepsy. NCSE
(non convulsive status epilepticus) can occur in about 8% of patients and
should be carefully excluded in children whose seizures/mental status
deteriorate on treatment(79).
It is used in an initial daily dose 0.1 mg/kg TID;
increased weekly by 0.1 mg/kg; maximum daily dose 0.4 and 0.7 mg/kg (uninduced
and induced, respectively). In children over 12 years, it can be initiated
at 4 mg/day; total daily dose increased by 4 mg in week 2 (divided doses);
then increased by 4 to 8 mg/day (divided doses) each week until clinical
response is achieved or to a maximum daily dose of 32 mg/d is reached.
12. Ketogenic Diet in Epilepsy
The ketogenic diet (KD) is a stringently controlled
high fat and low protein/carbohydrate diet given with/without a restricted
fluid intake to maintain ketosis on a long term basis(80). It has been
shown that it is more efficacious than newer AEDs in controlling
refractory seizures(81) and is more cost effective. It can be used with
both non-vegetarian and vegetarian diets at any age and for all types of
seizures. It has significant improvements in hyperactivity and aggression
in almost all patients(80,81). Hence, it should be tried in all children
above the age of 1 year with drug-resistant epilepsy, especially those who
are not a surgical candidates or where surgery cannot be performed due to
availability/affordability issues. Referral to centers providing the KD
should be considered once adequate trials of three AEDs have failed,
suggestive of pharmacoresistant epilepsy(82). Adverse effects(83) include
GI disturbances, acidosis, increased susceptibility to infections,
drowsiness, weight loss, nutritional deficiencies and rarely, renal
calculi and pancreatitis. Most of these occur early in the diet and should
be carefully monitored. The diet should be considered a failure if there
is no benefit in 3-6 months and it should be discontinued after this time.
In responders, it should be continued for 2-3 year after which it is
gradually tapered.
13. Surgically Remediable Syndromes
All infants and children with refractory partial or
generalized epilepsy should be referred as early as possible to a
comprehensive epilepsy center for possible surgical evaluation. This
process should be expedited if there is an imaging documented unilateral
lesion or if the epilepsy is having significant effects on the child’s
development.
Ideal surgically remediable syndromes(84) include:
• Hemispheric epilepsies with pre-existing
contralateral hemiplegias/visual field defects caused by large
unilateral gliotic lesions/atrophy, Rasmussen’s encephalitis,
hemispheric dys-plasias etc, where hemispherectomy/hemis-pherotomies
could offer a possible surgical cure.
• Discrete lesions without involvement of functional
motor, visual and language cortex, where a lesionectomy will often
result in a complete cure. Common lesions would include developmental
tumors, cortical dysplasias, AVMs etc. Sometimes lesions like large
dysplasias/infarcts may need lobectomies/multi-lobar resections.
• Mesial temporal lobe epilepsy caused often by
hippocampal sclerosis is not uncommon in teenagers and is amenable to an
anterior temporal lobectomy.
• Drop attacks with injuries respond well to corpus
callosotomy and should be offered as a palliative procedure.
14. Refractory Epilepsy
Refractory epilepsy in childhood can be defined as
epilepsy which is uncontrolled despite adequate trials of three first line
AEDs(82) and when it disrupts developmental progress or normal childhood
activity(85). When faced with a child with uncontrolled epilepsy, always
try and confirm whether the diagnosis is correct. Often non-epileptic
conditions may be confused as seizures (see above). Also the type of
seizure and if possible, a correct diagnosis of the specific epilepsy
syndrome may facilitate correct choice of drug.
Errors in management must be looked for as
pseudo-intractability often results from an inadequate dose, irrational
polytherapy or wrong choice of AED e.g. carbamazepine for absence
seizures(86). Every effort should be made to keep a seizure diary and see
if a specific AED is actually helping or in some cases worsening the
seizures e.g. carbamazepine/oxcarbazine may worsen and sometimes even
induce absence/myoclonic seizures(87,88).
It is best to refer intractable epilepsy early to a
tertiary center for appropriate evaluation (including high-end MRI using
standardized epilepsy proto-cols, video EEG etc) as well as to get
guidance on management options like newer AEDs, the ketogenic diet and
surgery.
15. Catastrophic Epilepsies in Infancy and
Early Childhood
West syndrome, symptomatic generalized epilepsies like
the Lennox-Gastaut syndrome and many other lesional partial epilepsies
starting in infancy, which may have fairly rapid effects on the developing
brain with high risk are appropriately labeled as the catastrophic
epilepsies. These often require fairly detailed knowledge and expertise in
both evaluation and management and are best referred to a specialized
centre where pediatric neurologist or epileptologist is available for
specialized care.
West syndrome (WS)
Early recognition needs taking a detailed history of
the jerks with an emphasis on when they occur (usually on awakening in
clusters lasting few minutes)(89). Typically, they are described as "jhatka"
in Hindi, "dachakte" in Marathi and "chamke" in Gujrathi. There is often
loss of eye contact and social smile, which should be carefully looked
for. This may sometimes precede spasm onset by days or weeks.
A detailed history of preceding perinatal events
(hypoxic-ischemic encephalopathy, neonatal hypo-glycemia etc),
developmental milestones, examination of the skin for stigmata of tuberous
sclerosis, head circumference measurement and careful neurologic/developmental
examination looking for deficits/delays will help to differentiate
cryptogenic vs. symptomatic spasms.
An EEG should be done to confirm the diagnosis, though
the characteristic pattern of hypsarrhythmia is not mandatory for
diagnosis(89,90). Moreover, an experienced electroencephalographer should
be available for interpretation.
If the history and examination do not reveal an obvious
etiology, an MRI preferably with special techniques to look for
malformations of cortical development should be undertaken. Metabolic
tests are usually unhelpful and should be done only in selected
infants(90), where there is a high suspicion for a neurometabolic
condition e.g. consanguinity, positive family history, etc.
Steroids are the drugs of first choice in all cases of
West syndrome(90,91), especially so in cryptogenic WS, except in tuberous
sclerosis. ACTH is preferred over oral steroids(90,91). Oral prednisolone
is given in doses of 2-4 mg/kg(91) or natural ACTH in 30-40 units/day (3-6
U/kg) for 2 weeks, with rapid taper over the next 2 weeks. Rapid control
of the spasms within 1 month of onset is associated with rapid
developmental gains (VPA could be continued after steroids in symptomatic
West syndrome).
Vigabatrin is preferred in TS as first choice(90,91)
and in steroid failures in the others (taking into account
availability/affordability issues). It should be used for a period of 3-6
months only, due to the fear of visual field defects.
Nitrazepam/high dose VPA/Topiramate can be used as
alternatives. The ketogenic diet and surgery in selected lesional cases
are other alternatives.
Lennox Gastaut syndrome
Any toddler, who has epileptic drop attacks and is
delayed or has arrest in development, should be considered to have
LGS(89). Usually other types of seizures are also present (like atypical
absence and brief tonic seizures in sleep).
An MRI is essential in case no obvious cause is
identified on history and examination. An EEG should be performed to
confirm the diagnosis though the typical slow spike-wave paroxysms are not
mandatory for diagnosis(89). It is mandatory for diagnosis of
non-convulsive status epilepticus, which often occurs in LGS and manifests
as decreased responsiveness, drooling and regression of milestones lasting
hours to days(89).
VPA and CLB should be used initially. LTG(92) or
TPM(93) should be added in case of continuing seizures. CBZ and OXZ should
be avoided.
The ketogenic diet should be used early, if available.
Helmets should be worn to prevent head injury. It is best to refer such
children to a tertiary epilepsy center to manage these complicated
patients.
Dravet’s syndrome (Severe myoclonic epilepsy of
infancy)
One should think of this syndrome in normal infants
with onset of refractory febrile/afebrile, focal/ generalized seizures in
the first year of life(94). They often present as refractory febrile or
afebrile status epilepticus (SE)(94) which often lasts several hours. Over
the next 2-3 years, delayed language development, autistic features and
later, gait difficulties become evident. Myoclonic and absence seizures,
often photosensitive usually become prominent after the first year, though
they are not mandatory for diagnosis (93). VPA, CLB and TPM(71) are drugs
most likely to help prevent SE, though a full remission is unlikely to
occur. LTG and CBZ regularly worsen these seizures and therefore should be
avoided in all febrile seizures in infancy, even those which are
clinically focal. Once this condition is considered, it is best to refer
to a tertiary care center for further evaluation and management.
16. Refractory Epilepsies in Older Children
and Adolescents
Mesial Temporal Lobe Epilepsy
Clinical recognition of mesial temporal lobe epilepsy (MTLE)
is important as it is the most common refractory epilepsy syndrome in the
older child/teenager. It is often caused by hippocampal sclerosis, though
other etiologies like cortical dysplasia, tumors and vascular
malformations may also underlie it. It presents as complex partial
seizures with an aura of fear or epigastric sensation followed by
unresponsiveness, automatisms and later secondary generalization. The
patients have often had febrile seizures (often febrile status epilepticus)
in the past. The complex partial seizures present several years later and
over time become refractory.
Sleep deprived EEGs with special electrodes are often
needed in these cases and should be also done in specialized centers. High
quality MRIs need to be done to diagnose hippocampal sclerosis and where
MTLE is suspected, it is better to refer the child for an MRI through a
specialized center to avoid dual costs. In many children, conventional
AEDs like CBZ, PHT and newer AEDs like OXZ and TPM are effective for a
while in controlling seizures. However a large number become resistant to
AEDs and there is a progressive cognitive, behavioural and memory
impairment, if the epilepsy remains uncontrolled. Early surgery in the
form of anterior temporal lobectomy is significantly more effective than
best medical treatment in adults(95).
Epilepsia Partialis Continua
Epilepsia partialis continua should be suspected when
focal, fairly constant myoclonic/clonic jerks involve one or more parts of
the body (face/limb/tongue) only unilateraly(96). In most children,
progressive, presumably immune-mediated encephalitis, Rasmussen’s
encephalitis underlies this disorder. Over time, a progressive hemiplegia
with deterioration in cognition and behaviour is usual, as the epilepsy is
resistant to all AEDs(96).
Investigation should include a MRI which is initially
often normal but shows progressive hemi-atrophy and unilateral signal
changes. EEGs are also helpful though they may be deceptively show absence
of abnormalities. Only focal background disturbances may be seen. Till a
hemiplegia develops immune therapies like steroids and IVIG can be used.
Treatment is again primarily surgical(96) and a
hemispheric resection/disconnection are the pro-cedure that seems to
benefit a large number. The only drawback is that a permanent motor/visual
field defect is invariant after a hemispherectomy. Hence, this procedure
becomes difficult in children who still have good function of the limbs.
17. Epilepsies and Cognition
Cognitive deterioration, academic underachieve-ment and
behavioral problems are common co morbidities in children with chronic
epilepsy(97-99). Uncontrolled seizures and worsening of the EEG with
increasing epileptiform abnormalities are more likely to be responsible
for cognitive deterioration, than any AED(100).
All children with epilepsy should be screened with a
simple child behavior checklist(101) consisting of questions directed
towards mood, behavior and school performance. Some children merit a more
formal neuropsychological examination.
Sensitization of parents and teachers regarding
associated co-morbidities and early referral for psycho-educational
evaluation and special education is useful.
All attempts to switch to monotherapy should be done to
reduce AED induced behaviour / cognitive effects.
It is important to recognize rare but important
syndromes like the Landau-Kleffner syndrome (LKS) and Continuous
Spike-Wave in Slow-Wave Sleep (CSWS)(102,103). LKS presents as predominant
language deterioration in a previously normal child who may or may not
have clinical seizures. Initially the child may stop responding when
called and may appear to be deaf; there may be school performance as well
as behavior deterioration. CSWS is a more global disturbance with a frank
dementia and autism. Both these syndromes are presumably causally
associated with a continuous epileptic discharges in non-REM sleep –
electrical status in slow wave sleep (ESES). Hence, any child with or
without epilepsy, who has cognitive, language and behavioural
deterioration should have an awake and more importantly sleep EEG to
establish the diagnosis. Evaluation and management of these complex
syndromes need referral to specialized epilepsy centers.
18. Status Epilepticus
Children with seizure clusters are at increased risk of
SE. Use of oral CLB or DZP for 2 days may be beneficial in decreasing this
risk. A child who is brought to the physician from an extramural setting
still convulsing should be considered to be in SE; the minimum time for
this definition of SE is regarded as >5 minutes(104). There is an
increased risk of irreversible neuronal injury after 30 minutes of
convulsive status(105). A management algori-thm(106-110) is provided for
status epilepticus in Fig 2.
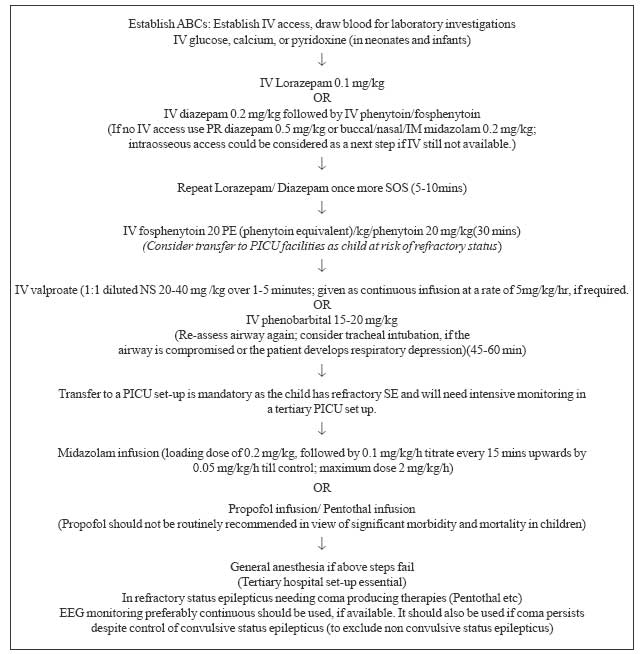 |
|
Fig. 2 Management
algorithm for status epilepticus. |
Competing interests: None stated.
Funding: Sanofi Aventis India Ltd provided the
funding for conducting the meeting.
|
Annexure
Writing Committee: Vrajesh Udani,
Consultant in pediatric neurology and epilepsy, PD Hinduja Hospital
and Research Centre, Mumbai; Neeta Naik, Consultant in
pediatric epilepsy, Epilepsy Research Centre for Children, Mumbai.
Chairpersons: Nitin Shah, President IAP 2006;
Deepak Ugra, Secretary IAP 2006.
Conveners: V Udani, N Naik.
List of experts: Surekha Rajyadhyaksha,
Bharati Vidyapeeth Medical College and Hospital, Pune; Veena
Kalra, All India Institute of Medical Sciences, New Delhi;
Nagabhushana Rao Potharaju, Osmania Medical College and Osmania
General Hospital; Pratibha Singhi, PGIMR, Chandigarh; KN
Shah, Wadia Children’s Hospital, Lilavati Hospital, and Bhatia
Hospital, Mumbai; Nandan Yardi, Member, Commission on
treatment Strategies ,ILAE, Pune; JMK Murthy, Nizam’s
Institute of Medical Sciences, Hyderabad; Margi Desai, Saifee
Hospital, Mumbai; Meher Ursekar, Wellspring Jankharia
Imaging, Mumbai; Anaita Hegde, Jaslok Hospital & Research
Centre and Wadia Childrens Hospital, Mumbai; G. Kumaresan,
Institute of Child Health and Hospital for Children, Chennai;
Satinder Aneja, Lady Harding Medical College and Associated
Kalawati Saran Children’s Hospital, New Delhi; Vrajesh Udani,
PD Hinduja Hospital and Research Centre and GMC & JJ Group of
Hospitals, Mumbai; Jayanti Mani, Kokilaben Dhirubhai Ambani
Hospital, Mumbai. Neeta Naik, Epilepsy Research Centre for
Children, Mumbai; Rachna Gupta, CHL Apollo Hospital, Indore;
GR Passi, Choithram Hospital & Research Centre, Indore;
Arijit Chattopadhyay. Institute of Child Health, Kolkata; J
Nathan, Shushrusha Hospital & Research Centre, Mumbai; Milind
Sankhe, P D Hinduja Hospital and Research Centre, Mumbai; Soono
Udani, PD Hinduja National Hospital and Research Centre and Grant
Medical College and JJ Group of Hospitals. |
References
1. Pal DK. Epilepsy control in the 21st century: leave
no child behind. Epilepsia 2003; 44: 273-275.
2. Shiffman RN, Shekelle P, Overhage JM, Slutsky J,
Grimshaw J, Deshpande AM. Standardized Repor-ting of Clinical Practice
Guidelines: A proposal from the Conference on Guidelines Standardi-zation.
Ann Intern Med 2003; 139: 493-498.
3. Mizrahi EM, Watanabe K. Symptomatic neonatal
seizures: In: Roger J, Bureau M. Epileptic Synd-romes in Infancy,
Childhood and Adolescence, 4th ed. London: John Libbey; 2005. p. 16-17.
4. Bauder F, Wohlrab G, Schmitt B. Neonatal seizures:
eyes open or closed. Epilepsia 2007; 48: 394-396.
5. Co JP, Elia M, Engel J Jr, Guerrini R, Mizrahi EM,
Moshé SL. Proposal of an algorithm for diagnosis and treatment of neonatal
seizures in developing countries. Epilepsia 2007; 48: 1158-1164.
6. Sankar MJ, Agarwal R, Aggarwal R, Deorari AK, Paul
VK. Seizures in the newborn. Indian J Pediatr 2008; 75: 149-155.
7. Castro Conde JR, Hernández Borges AA, Doménech
Martínez E, González Campo C, Perera Soler R. Midazolam in neonatal
seizures with no response to phenobarbital. Neurology 2005; 64: 876-879.
8. Huang CC, Chang YC, Wang ST. Acute symptomatic
seizure disorders in young children-a population study in southern Taiwan.
Epilepsia 1998; 39: 960-964.
9. Murthy JM, Yangala R. Acute symptomatic seizures -
incidence and etiological spectrum: a hospital-based study from South
India. Seizure 1999; 8: 162-165.
10. Balasubramanian S, Shivbalan S, Kumar PS.
Hypocalcemia due to vitamin D deficiency in exclusively breastfed infants.
Indian Pediatr 2006; 43: 247-251.
11. Agrawal A, Timothy J, Pandit L, Manju M.
Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg 2006; 108:
433-439.
12. Singhi PD, Srinivas M. Febrile seizures. Indian
Pediatr 2001; 38: 733 –740.
13. Sadleir LG, Scheffer IE. Febrile seizures. BMJ
2007; 334: 307-311.
14. Baumann RJ, Duffner PK. Treatment of children with
simple febrile seizures: the AAP practice parameter. American Academy of
Pediatrics. Pediatr Neurol 2000; 23: 11-17.
15. Bhattacharyya M, Kalra V, Gulati S. Intranasal
midazolam vs. rectal diazepam in acute childhood seizures. Pediatr Neurol
2006; 34: 355-359.
16. ILAE Neuroimaging Commission. ILAE Neuroimaging
Commission Recommendations for Neuroimaging of Patients with Epilepsy.
Epilepsia 1997; 38: 1-2.
17. Singhi P, Singhi S. Neurocysticercosis in children.
J Child Neurol 2004; 19: 482-492.
18. Wasay M, Kheleani BA, Moolani MK, Zaheer J, Pui M,
Hasan S, et al. Brain CT and MRI findings in 100 consecutive
patients with intracranial tuberculoma. J Neuroimaging 2003; 13: 240-247.
19. Pretell EJ, Martinot C Jr, Garcia HH, Alvarado M,
Bustos JA, Martinot C. Cysticercosis Working Group in Peru. Differential
diagnosis between cerebral tuberculosis and neurocysticercosis by magnetic
resonance spectroscopy. J Comput Assist Tomogr 2005; 29: 112-114.
20. Del Brutto OH, Roos KL, Coffey CS, Garcia HH.
Meta-analysis: Cysticidal drugs for neuro-cysticercosis: albendazole and
praziquantel. Annals Int Med 2006; 1: 43-51.
21. Singhi P, Dayal D, Khandelwal N. One week versus
four weeks of albendazole therapy for neurocysticercosis in children: a
randomized, placebo-controlled double blind trial. Pediatr Infect Dis J
2003; 22: 268-272.
22. Thussu A, Arora A, Prabhakar S, Lal V, Sawhney IM.
Acute symptomatic seizures due to single CT lesions: how long to treat
with antiepileptic drugs? Neurol India 2002; 50: 141-144.
23. Camfield P, Camfield C. Epileptic syndromes in
childhood: clinical features, outcomes, and treatment. Epilepsia 2002; 43:
27-32.
24. Commission on Classification and Terminology of the
International League against Epilepsy: Proposal for revised classification
of epilepsies and epileptic syndromes. Epilepsia 1989; 30: 389-399.
25. Panayiotopoulos CP. Early-onset benign childhood
occipital seizure susceptibility syndrome: a syndrome to recognize.
Epilepsia 1999; 40: 621-630.
26. Prats JM, Garaizar C, García-Nieto ML, Madoz P.
Antiepileptic drugs and atypical evolution of idiopathic partial epilepsy.
Pediatr Neurol 1998; 18: 402-406.
27. Frank LM, Enlow T, Holmes GL, Manasco P, Concannon
S, Chen C, et al. Lamictal (lamotrigine) monotherapy for typical
absence seizures in children. Epilepsia 1999; 40: 973-979.
28. Reutens DC, Berkovic SE. Idiopathic generalized
epilepsy of adolescence: are the syndromes clinically distinct? Neurology
1995; 45: 1469-1476.
29. Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R,
Baker GA, Chadwick DW, et al. SANAD Study group: The SANAD study of
effectiveness of valproate, lamotrigine, or topiramate for generalised and
unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet
2007; 369: 1016-1026.
30. Deuschl G, Eisen A. Recommendations for the
practice of clinical neurophysiology: Guidelines of the International
Federation of Clinical Neurophysiology. Electroencephalography and
Clinical Neurophysiology. Amsterdam, Oxford: Elsevier; 1999.
31. American Clinical Neurophysiology Society.
Guidelines 1, 2: Minimum technical requirements for performing clinical
electroencephalography. J Clin Neurophysiol 2006; 23: 86-91.
32. Wright NB. Imaging in epilepsy: a paediatric
perspective. Br J Radiol 2000; 74: 575-589.
33. Uldall P, Alving J, Hansen LK, Kibaek M, Buchholt
J. The misdiagnosis of epilepsy in children admitted to a tertiary
epilepsy centre with paroxysmal events. Arch Dis Child 2006; 91: 219-221.
34. Hirtz D, Berg A, Bettis D. Practice parameter:
treatment of the child with a first unprovoked seizure: Report of the
Quality Standards Subcommittee of the American Academy of Neurology and
the Practice Committee of the Child Neurology Society. Neurology 2003; 60:
166-175.
35. Stroink H, Geerts AT, van Donselaar CA, Peters AC,
Brouwer OF, Peeters EA, et al. Status epilepticus in children with
epilepsy: Dutch study of epilepsy in childhood. Epilepsia 2007; 48:
1708-1715.
36. Holsti M, Sill BL, Firth SD, Filloux FM, Joyce SM,
Furnival RA. Prehospital intranasal midazolam for the treatment of
pediatric seizures. Pediatr Emerg Care 2007; 23: 148-153.
37. Brodie MJ. Medical therapy of epilepsy: when to
initiate and combine. J Neurol 2005; 252: 125-130.
38. O’Dells. Initiation and discontinuation of AEDs.
Neurol Clin 2001; 19: 289-311.
39. Bialer M. Extended-release formulations for the
treatment of epilepsy. CNS Drugs 2007; 21: 765-774.
40. De Silva M, MacArdle B, McGowan M, Hughes E,
Stewart J, Neville BG. Randomised comparative monotherapy trial of
phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly
diagnosed childhood epilepsy. Lancet 1996; 347: 709-713.
41. Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC,
Glauser TA, Johannessen SI. Antiepileptic drugs-best practice guidelines
for therapeutic drug monitoring: a position paper by the subcommission on
therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies.
Epilepsia 2008; 49: 1239-1276.
42. Specchio LM, Beghi E. Should antiepileptic drugs be
withdrawn in seizure-free patients? CNS Drugs 2004; 18: 201-212.
43. Matricardi M, Brinciotti M, Benedetti P. Outcome
after discontinuation of antiepileptic drug therapy in children with
epilepsy. Epilepsia 1989; 30: 582-589.
44. Serra JG, Montenegro MA, Guerreiro MM.
Antiepileptic drug withdrawal in childhood: does the duration of tapering
off matter for seizure recurrence? J Child Neurol 2005; 20: 624-626.
45. Ranganathan LN, Ramaratnam S. Rapid versus slow
withdrawal of antiepileptic drugs. Cochrane Database Syst Rev 2006; 19:
CD005003.
46. Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR,
Ivacko JA. Neonatal seizures: multicenter variability in current treatment
practices. Pediatr Neurol 2007; 37: 85-90.
47. Kwan P, Brodie MJ. Phenobarbital for the treatment
of epilepsy in the 21st century: a critical review. Epilepsia 2004; 45:
1141-1149.
48. Camfield C, Camfield P. Management guidelines for
children with idiopathic generalized epilepsy. Epilepsia 2005; 46:
112-116.
49. Guerrini R. Valproate as a mainstay of therapy for
pediatric epilepsy. Paediatr Drugs 2006; 8: 113-129.
50. Verrotti A, Greco R, Latini G, Chiarelli F.
Endocrine and metabolic changes in epileptic patients receiving valproic
acid. J Pediatr Endocrinol Metab 2005; 18: 423-430.
51. Schulpis KH, Karikas GA, Tjamouranis J, Regoutas S,
Tsakiris S. Low serum biotinidase activity in children with valproic acid
monotherapy. Epilepsia 2001; 42: 1359-1362.
52. Russell S. Carnitine as an antidote for acute
valproate toxicity in children. Curr Opin Pediatr 2007; 19: 206-210.
53. Wheless JW, Clarke DF, Carpenter D. Treatment of
pediatric epilepsy: expert opinion. J Child Neurol 2005; 20: S1-56.
54. Eeg-Olofsson O, Nilsson HL, Tonnby B, Arvidsson J,
Grahn PA, Gylje H, et al. Diurnal variation of carbamazepine and
carbamazepine-10,11-epoxide in plasma and saliva in children with
epilepsy: a comparison between conventional and slow-release formulations.
J Child Neurol 1990; 5: 159-165.
55. Ryan SW, Forsythe I, Hartley R, Haworth M, Bowmer
CJ. Slow release carbamazepine in treatment of poorly controlled seizures.
Arch Dis Child 1990; 65: 930-935.
56. Parmeggiani A, Fraticelli E, Rossi PG. Exacerbation
of epileptic seizures by carba-mazepine: report of 10 cases. Seizure 1998;
7: 479-483.
57. Bajaj AS. Intermittent clobazam in febrile
seizures: an Indian experience. Pediat Neurol 2005; 3: 19-23.
58. Chellam K. Intermittent clobazam therapy in febrile
seizures. Indian J Pediatr 2005; 72: 31-33.
59. Himizu H. Use of clobazam for the treatment of
refractory complex partial seizures. Seizure 2003; 12: 282-286.
60. Munn R, Farrell K. Open study of clobazam in
refractory epilepsy. Pediatr Neurol 1993; 9: 465-469.
61. Satishchandra P. Long term use of clobazam in the
management of intractable epilepsy: a prospective study. Neurol Annals
1998; 46: 284-287.
62. Canadian Clobazam Cooperative Group. Clobazam in
treatment of refractory epilepsy: the Canadian experience. A retrospective
study. Epilepsia 1991; 32: 407-416.
63. Guerreiro MM, Vigonius U, Pohlmann H, de Manreza
ML, Fejerman N, Antoniuk SA, et al. A double-blind controlled
clinical trial of oxcarbazepine versus phenytoin in children and
adolescents with epilepsy. Epilepsy Res 1997; 27: 205-213.
64. French JA, Kanner AM, Bautista J. Efficacy and
tolerability of the new antiepileptic drugs II. Treatment of refractory
epilepsy. Report of the therapeutics and technology assessment
sub-committee and quality standards subcommittee of the American Academy
of Neurology and the American Epilepsy Society. Neurology 2004; 62:
1261-1273.
65. Pina Garza JE, Espinoza R, Nordli D, Bennett DA,
Spirito S, Stites TE, et al. Oxcarbazepine adjunctive therapy in
infants and young children with partial seizures. Neurology 2005; 65:
1370-1375.
66. Albani F, Grassi B, Ferrara R. Immediate
(overnight) switching from carbamazepine to oxcarbamazepine monotherapy is
equivalent to a progressive switch. Seizure 2004; 13: 254-263.
67. French JA, Kanner AM, Bautista J, Abou-Khalil B,
Browne T, Harden CL, et al. Therapeutics and Technology Assessment
Subcommittee of the American Academy of Neurology; Quality Standards
Subcommittee of the American Academy of Neurology; American Epilepsy
Society. Efficacy and tolerability of the new antiepileptic drugs I:
treatment of new onset epilepsy.: report of the Therapeutics and
Technology Assessment Subcommittee of the American Academy of Neurology;
Quality Standards Subcommittee of the American Academy of Neurology;
American Epilepsy Society. Neurology 2004; 62: 1252-1260.
68. Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R,
Baker GA, Chadwick DW. The SANAD study of effectiveness of carbamazepine,
gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of
partial epilepsy: an unblinded randomised controlled trial. Lancet 2007;
369: 1000-1015.
69. Glauser TA. Topiramate in the catastrophic
epilepsies of childhood. J Child Neurol 2000; 15: 14-21.
70. Hosain SA. Topiramate for the treatment of
infantile spasms. J Child Neurol 2006; 21: 17-19.
71. Kröll-Seger J, Portilla P, Dulac O, Chiron C.
Topiramate in the treatment of highly refractory patients with Dravet
syndrome. Neuropediatrics 2006; 37: 325-329.
72. Albsoul-Younes AM. Topiramate slow dose titration:
improved efficacy and tolerability. Pediatr Neurol 2004; 31: 349-352.
73. Philippi H. Topiramate and metabolic acidosis in
infants and toddlers. Epilepsia 2002; 43: 744-747.
74. Ben-Zeev B, Watemberg N, Augarten A, Brand N, Yahav
Y, Efrati O, et al. Oligohydrosis and hyperthermia: pilot study of
novel topiramate adverse effect. J Child Neurol 2003; 18: 254-257.
75. Glauser TA, Ayala R, Elterman RD, Mitchell WG, Van
Orman CB, Gauer LJ, et al; on behalf of the N159 Study Group.
Double-blind placebo-controlled trial of adjunctive levetiracetam in
pediatric partial seizures. Neurology 2006; 66: 1654-1660.
76. Grosso S, Franzoni E, Coppola G, Lannetti P,
Verrotti A, Cordelli DM, et al. Efficacy and safety of
levetiracetam: An add-on trial in children with refractory epilepsy.
Seizure 2005; 14: 248-253.
77. Crest C, Dupont S, Leguern E, Adam C, Baulac M.
Levetiracetam in progressive myoclonic epilepsy: an exploratory study in 9
patients. Neurology 2004; 62: 640-643.
78. Pellock JM. Tiagabine (gabitril) experience in
children. Epilepsia 2001; 42: 49-51.
79. Balslev T, Uldall P, Buchholt J. Provocation of
non-convulsive status epilepticus by tiagabine in three adolescent
patients. Eur J Paediatr Neurol 2000; 4: 169-170.
80. Freeman JM, Kossoff EH, Hartman AL. The ketogenic
diet: one decade later. Pediatrics 2007; 119: 535-543.
81. Lefevre F, Aronson N. Ketogenic diet for the
treatment of refractory epilepsy in children: a systemic review of
efficacy. Pediatrics 2000; 105: e46.
82. Camfield PR, Camfield CS. Antiepileptic drug
therapy: when is epilepsy truly intractable? Epilepsia 1996; 37: 60-65.
83. Kang HC. Early and late onset complications of the
ketogenic diet for intractable epilepsy. Epilepsia 2004; 45: 1116-1123.
84. Cherian PJ, Radhakrishnan K. Selection of ideal
candidates for epilepsy surgery in developing countries. Neurol India
2002; 50: 11-16.
85. Udani VP, Dharnidharkari V, Nair A, Oka M.
Difficult to control epilepsy in childhood—a long term study of 123 cases.
Indian Pediatr 1993; 30: 1199-1206.
86. Gayatri NA, Livingston JH. Aggravation of epilepsy
by anti-epileptic drugs. Dev Med Child Neurol 2006; 48: 394-398.
87. Kochen S, Giagante B, Oddo S. Spike-and-wave
complexes and seizure exacerbation caused by carbamazepine. Eur J Neurol
2002; 9: 41-47.
88. Vendrame M, Khurana DS, Cruz M, Melvin J, Valencia
I, Legido A. Aggravation of seizures and/or EEG features in children
treated with oxcarbazepine monotherapy. Epilepsia 2007; 48: 2116-2120.
89. Shields WD. Diagnosis of infantile spasms, Lennox-Gastaut
syndrome, and progressive myoclonic epilepsy. Epilepsia 2004; 45: 2-4.
90. Mackay MT, Weiss SK, Adams-Webber T, Ashwal S,
Stephens D, Ballaban-Gill K, et al. American Academy of Neurology;
Child Neurology Society Practice Parameter: Medical treatment of infantile
spasms: report of the American Academy of Neurology and the Child
Neurology Society. Neurology 2004; 62: 1668-1681.
91. Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy
CR, Newton RW; United Kingdom Infantile Spasms Study. The United Kingdom
Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin
on developmental and epilepsy outcomes to age 14 months: a multicentre
randomized trial. Lancet Neurol 2005; 4: 712-717.
92. Eriksson AS, Nergardh A, Hoppu K. The efficacy of
lamotrigine in children and adolescents with refractory generalized
epilepsy: a randomized, double-blind, crossover study. Epilepsia 1998; 39:
495-501.
93. Sachdeo RC, Glauser TA, Ritter F, Reife R, Lim P,
Pledger G. A double-blind, randomized trial of topiramate in Lennox-Gastaut
syndrome. Topira-mate YL Study Group. Neurology 1999; 52: 1882-1887.
94. Korff CM, Nordli DR Jr. Epilepsy syndromes in
infancy. Pediatr Neurol 2006; 34: 253-263.
95. Wiebe S, Blume WT, Girvin JP, Eliasziw M.
Effectiveness and efficiency of surgery for temporal lobe epilepsy study
group; A randomized, controlled trial of surgery for temporal-lobe
epilepsy. N Engl J Med 2001; 345: 311-318.
96. Freeman JM. Rasmussen’s syndrome: progressive
autoimmune multi-focal encephalopathy. Pediatr Neurol 2005; 32: 295-299.
97. Aldenkamp AP, Weber B, Overweg-Plandsoen WC, Reijs
R, van Mil S. Educational under-achievement in children with epilepsy: a
model to predict the effects of epilepsy on educational achievement. J
Child Neurol 2005; 20: 175-180.
98. Sanchez-Carpintero R, Neville BG. Attention ability
in children with epilepsy. Epilepsia 2003; 44: 1340-1349.
99. Nordli DR Jr. The management of epilepsy in
children: cognitive and behavioral side effects. Rev Neurol Dis 2004; 1:
4-9.
100. Lagae L. Cognitive side effects of anti-epileptic
drugs. The relevance in childhood epilepsy. Seizure 2006; 15: 227-234.
101. Achenbach TM, Ruffle TM. The Child Behavior
Checklist and related forms for assessing behavioral/emotional problems
and competencies. Pediatr Rev 2000; 21: 265-271.
102. Van Rijckevorsel K. Cognitive problems related to
epilepsy syndromes, especially malignant epilepsies. Seizure 2006; 15:
227-234.
103. Beaumanoir A, Bureau M, Deonna T, Mira L,
Tassinari CA. Continuous Spikes and Waves, During Slow Sleep Electrical
Status Epilepticus during Slow Sleep. London: John Libbey; 1995.
104. Lowenstein DH, Bleck T, Macdonald RL. It’s time to
revise the definition of status epilepticus. Epilepsia 1999; 40: 120-122.
105. Fountain NB. Status epilepticus: risk factors and
complications. Epilepsia 2000; 41: 23-30.
106. Appleton R, Martland T, Phillips B. Drug
management for acute tonic-clonic convulsions including convulsive status
epilepticus in children. Cochrane Database Syst Rev 2002: 4: CD001905.
107. Prasad K, Al-Roomi K, Krishnan PR, Sequeira R.
Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev
2005; 4: CD003723.
108. Treiman DM, Meyers PD, Walton NY, Collins JF,
Colling C, Rowan AJ, et al. A comparison of four treatments for
generalized convulsive status epilepticus. Veterans Affairs Status
Epilepticus Cooperative Study Group. N Engl J Med 1998; 339: 792-798.
109. Mehta V, Singhi P, Singhi S. Intravenous sodium
valproate versus diazepam infusion for the control of refractory status
epilepticus in children: a randomized controlled trial. J Child Neurol
2007; 22: 1191-1197.
110. Niermeijer JM, Uiterwaal CS, Van Donselaar CA.
Propofol in status epilepticus: little evidence, many dangers? J Neurol
2003; 250: 1237-1240.
|
|
|
 |
|

