|
|
|
Indian Pediatr 2016;53: 319-327 |
 |
Approach to Constipation in Children
|
|
Ujjal Poddar
From Department of Pediatric Gastroenterology, Sanjay
Gandhi Postgraduate Institute of Medical Sciences, Lucknow,
Uttar Pradesh, India.
Correspondence to: Dr Ujjal Poddar, Professor,
Department of Pediatric Gastroenterology, SGPGIMS, Lucknow 226
014,
Uttar Pradesh, India.
Email: [email protected]
|
|
Purpose: There is a scarcity of
literature, and prevalent misconceptions about constipation in India.
Methods: A literature search in
PubMed was conducted with regard to epidemiology, clinical features, and
management of constipation. Special emphasis was paid to functional
constipation and refractory constipation. English language studies
available full text over the last 25 years were considered and relevant
information was extracted.
Conclusions: Estimated prevalence
of constipation is 3% among toddlers and pre-school children worldwide
and 95%, of them are considered functional. A careful history and
thorough physical examination is all that is required to diagnose
functional constipation. Management includes disimpaction followed by
maintenance therapy with oral laxative, dietary modification and toilet
training. A close and regular follow-up is necessary for successful
treatment. In most of the cases laxative needs to be continued for
several months and sometimes years. Early withdrawal of laxative is the
commonest cause of recurrence. Refractory constipation is less common in
primary care set up. Radiological colon transit study is useful in
picking up Slow transit constipation. Antegrade continence enema plays
an important role in the management of slow transit constipation.
Key words: Functional constipation; Laxative;
Refractory; Slow transit constipation.
|
|
C
onstipation is a common problem in children and
it accounts for 3% of visits to general pediatric clinics and as many as
30% of visits to pediatric gastroenterologists in developed countries
[1]. There is very little information about its prevalence from
developing countries. However, some recent reports from south Asia have
suggested that it is not uncommon in Asia [2-4]. The common perception
in South Asia is that functional constipation is uncommon as diet here
is rich in fiber. Hence many children with constipation are subjected to
detailed investigations to rule out Hirschsprung disease. However,
whatever limited information we have from Asia shows that functional
constipation is the commonest type of constipation in Asia as well
[2-4]. The prevalence, etiology, pathogenesis, assessment and management
of constipation in children is discussed in this review.
Stool Pattern of Normal Infants
Normal variation in stool frequency and consistency
often leads to over-diagnosis of constipation especially in infants. Two
recent studies from the Europe (12,984 healthy children, 1-42 months
from UK [5] and 600 healthy infants from Netherlands [2]) have shown
that the median stool frequency at 1 month of age was 3 (0-9) per day
and it decreased significantly at 3 months of age to 2 (0-6) per day.
Moreover, there was a significant difference in stool frequency between
breastfed and formula-fed babies at 1 month of age [4 (0-9) vs. 1
(0-5) per day, respectively, P<0.01] but there was no difference
at 3 months of age [2 (0-6) vs. 1 (0-5) per day] [5,6]. Another
study from Turkey in 911 children aged 0 to 24 months has shown that the
median defecation frequency at 1 month of age was 6 per day and by 4-6
months of age it became 1 per day. The most interesting observation of
this study is that the stool frequency was <1 per day (once in 2-3 days
but soft stool) in 39.3% babies in 2-6 months of age [7]. Hence, while
considering constipation we should remember the normal variations of
stool frequency and consistency in healthy infants and variations as per
their feeding pattern (breast fed versus bottle fed).
Definition of Constipation
In view of wide variations in stool frequency and
consistency in normal healthy children, ROME III criteria [8,9] have
included other variables besides frequency of stool to define
constipation in children. As per ROME III criteria, functional
constipation is defined as presence of two or more of the following in
absence of any organic pathology and the duration should be at least one
month in <4 years of age, and at least once per week for at least 2
months in ³4
years of age; (i) two or less defecations per week, (ii)
at least one episode of fecal incontinence per week, (iii)
history of retentive posture or stool withholding maneuver, (iv)
history of painful or hard bowel movement, (v) presence of large
fecal mass in the rectum, (vi) history of large-diameter stools
that may obstruct the toilet. In children <4 years of age, the history
of retentive posture or stool withholding maneuver is being replaced by
history of excessive stool retention as retentive posture is difficult
to assess in younger children.
Prevalence
Constipation is a common problem in children and an
estimated prevalence of functional constipation is 3% worldwide
[1,10,11]. Though we do not have any prevalence data from Asia, in a
study from our center we reported 138 cases of constipation diagnosed
over a period of six years and 85% of them were functional [2]. In next
8 years (2007 to 2014), we managed another set of 330 children with
constipation and the proportion of functional constipation was 82% (270
of 330) [unpublished data]. Hence, constipation is not uncommon in the
Indian subcontinent. It is commonly seen among toddlers and preschool
children, and in 17% to 40% of cases, constipation starts in first year
of life [12,13].
Etiology
The common perception in South Asia is that
functional constipation is uncommon as diet in South Asia is rich in
fiber. In our study [2], we have shown that this perception is
incorrect. Constipation is quite common in India and functional
constipation is the commonest cause. Common causes of constipation in
children are given in Box I. In fact 95% cases are due to
functional and only 5% are due to some organic causes [14]. Among the
organic causes, Hirschsprung disease is the most common and important
cause [2].
|
BOX I Causes of Constipation in Children |
|
• Functional constipation of childhood
• Motility
related: Hirschsprung disease, myopathy
• Congenital
anomalies: Anal stenosis, anteriorly located anus, spinal
cord anomalies (meningomyelocele, myelomalacia, spina bifida)
• Neurological:
Cerebral palsy, mental retardation
•
Endocrine/metabolic: Hypothyroidism, renal tubular acidosis,
diabetes insipidus, hypercalcemia
• Drugs: Anticonvulsants, antipsychotic, codein
containing anti-diarrheal.
|
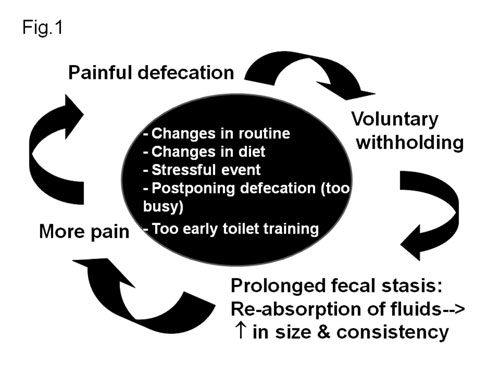 |
|
Fig. 1 Pathogenesis of functional
constipation.
|
Pathogenesis of functional constipation
(Fig. 1)
The initiating event in functional constipation is a
painful bowel movement which leads to voluntary withholding of stools by
the child who wants to avoid unpleasant defecation [15]. Events that
lead to initial painful defecation are change in routine like timing of
defecation or diet, stressful events, inter-current illness,
non-availability of toilets (travel etc.), child’s postponing defecation
because he or she is too busy (morning school), and forceful toilet
training (too early). All these events give rise to large, hard stool
and passage of such stool leads to stretching of the pain sensitive anal
canal, and that frightens the child. As a result of which the child
fearfully determines to avoid defecation by all of means. Such children
respond to the urge to defecate by contracting their external anal
sphincter and gluteal muscles, in an attempt to withhold stool.
Withholding of feces leads to prolonged fecal stasis in the rectum, with
resultant absorption of fluids and harder stools. Successive retention
of stools in rectum make them larger. As the cycle is repeated,
successively greater amounts of larger and harder stools are built up in
the rectum and passed with even greater pain accompanied by severe
"stool with-holding maneuvers". Thus a vicious cycle sets in (Fig.
1). These children develop a "stool-withholding maneuver" or
retentive posture which parents erroneously think it as an attempt to
defecate. They feel that the child is trying hard (straining) in an
attempt to pass stool when the child is actually trying his best to stop
it. In response to the urge, they refuse to sit on the toilet, rather
rise on their toes, hold their legs and buttocks stiffly and often rock
back and forth, holding on to a furniture, scream, turn red until a
bowel movement finally takes place. With time, such retentive behavior
becomes an automatic reaction. They often perform this while hiding in a
corner. Eventually, liquid stool from the proximal colon may percolate
around hard retained stool and pass per rectum involuntarily (fecal
incontinence). Sometimes this fecal incontinence is mistaken as
diarrhea. In fact almost 30% children with functional constipation
develop fecal incontinence [12]. Eventually, with more and more stasis,
the rectum becomes dilated and redundant, and the sensitivity of the
defecation reflex and the effectiveness of peristaltic contractions of
rectal muscles decrease. This is the stage when it becomes more
difficult to have a normal defecation due to fecal impaction.
Assessment of a Child with Constipation
A careful history and thorough physical examination
(including digital rectal examination) are all that is required to
diagnose functional constipation provided there are no "red flags" like
fever, vomiting, bloody diarrhea, failure to thrive, anal stenosis, and
tight empty rectum [16]. Abnormal physical findings, which help to
distinguish organic causes of constipation from functional, are failure
to thrive, lack of lumbo-sacral curve, sacral agenesis, flat buttock,
anteriorly displaced anus, tight and empty rectum, gush of liquid stool
and air on withdrawal of finger, absent anal wink and cremasteric
reflex. Features which differentiate Hirschsprung disease from
functional constipation are given in Table I. The most
important features in the history, which help to distinguish
Hirschsprung disease from functional consti-pation, are onset in first
month of life and delayed passage of meconium beyond 48 hours and
the most important examination finding is empty rectum on
digital rectal examination. It has been shown that 99% healthy,
term neonates and 50% babies with Hirschsprung disease pass meconium in
first 48h of life [17,18]. In fact, in a classical case of functional
constipation, no investigation is required to make the diagnosis. There
is no need to do barium enema in all cases of constipation to rule out
Hirschsprung disease. If the clinical suspicion of Hirschsprung disease
is strong (based on history of delayed passage of meconium and empty
rectum on digital rectal examination) then only one may consider getting
barium enema done. However, to diagnose Hirschsprung disease, rectal
biopsy is a must. The common mistake that leads to further confusion is
delayed film (24 hours) showing retention of barium which is a common
finding in functional constipation as well. The interpretation of barium
enema should be on the basis of reversal of recto-sigmoid ratio (sigmoid
becomes more dilated than rectum) and documentation of transition zone
and not on mere presence of barium in rectum after 24 hours (Fig.
2).
TABLE I Differences Between Functional Constipation and Hirschsprung Disease
|
Features |
Functional |
Hirschsprung
|
|
constipation |
disease |
|
Delayed passage of meconium |
None |
Common |
|
Onset |
After 2 years |
At birth |
|
Fecal incontinence |
Common |
Very rare |
|
History of fissure |
Common |
Rare |
|
Failure to thrive |
Uncommon |
Possible |
|
Enterocolitis |
None |
Possible |
|
Abdominal distension |
Rare |
Common |
|
Rectal examination |
Stool |
Empty |
|
Malnutrition |
None |
Possible |
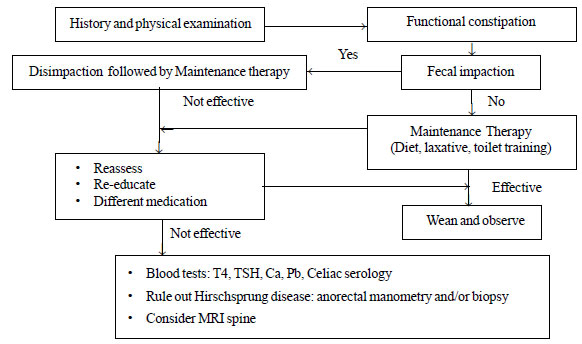 |
|
Fig. 3 Suggested approach to
functional constipation: modified from ESPGHAN recommendations.
|
Management
Most children with functional constipation get
benefited from a precise, well-organized treatment plan, which includes
cleaning of fecal retention, prevention of further retention and
promotion of regular bowel habits. The general approach includes the
following steps: (a) determine whether fecal impaction is
present, and treat the impaction if present, (b) initiate
maintenance treatment with oral laxative, dietary modification, toilet
training, and (c) close follow up and medication adjustment as
necessary [16]. Suggested approach to constipation is given in Fig.
3.
Disimpaction
First step in the management of constipation is to
decide whether the child has fecal impaction or not. This can be
accomplished by abdominal examination (in half of the cases hard fecal
mass or fecalith is palpable in the lower abdomen) [19], by digital
rectal examination (rectum is usually loaded with hard stools), or
rarely by abdominal X-ray. Routinely abdominal X-ray is
not required to detect fecal impaction. However, if the child refuses
rectal examination, if he/she is obese, or if there is a doubt about the
diagnosis of constipation then only an abdominal X-ray is
required to document excess fecal matter in the colon.
If there is fecal impaction (most of the children
with functional constipation do have), then the first step in the
management is disimpaction, means clearing or removal of retention from
the rectum. This can be achieved by oral or by rectal route. Oral route
is non-invasive, gives a sense of power to the child but compliance is a
problem. Polyethylene glycol (PEG) lavage solution is given orally
(1-1.5g/kg/day for 3-6 days) or by naso-gastric tube (25mL/kg/hour,
reconstituted PEG solution) until clear fluid is excreted through anus.
Adequate disimpaction means both output (stool) and input (lavage
solution) should be of same color in case of naso-gastric tube
disimpaction [16]. Successful disimpaction for home-based regimen (3-6
days) is defined as either empty or a small amount of soft stool on
rectal examination and resolution of the left lower quadrant mass if it
was there [20, 21].
Rectal approach (enema) is faster but invasive,
likely to add fear and discomfort that the child already has in relation
to defecation. This may aggravate defecation avoidance or retention
behavior and usually not preferred. However, if PEG is not available
then enema can be used for disimpaction (sodium phosphate enema [proctoclysis]:
2.5 mL/kg, maximum 133ml/dose for 3-6 days) [16]. In a retrospective
chart review of 223 children, Guest, et al. [22] have shown that
97% children treated with PEG were successfully disimpacted compared to
73% of those who received enemas and suppositories (P<0.001). In
a randomized controlled trial, Bekkali, et al. [20] have compared
6 days enemas with dioctylsulfosuccinate sodium (60 mL in <6 years and
120 mL in ³6
years) in 46 children with PEG in 44 children and showed that both were
equally effective for disimpaction. However, two retrospective studies
have shown that the reimpaction rate after initial disimpaction with
enemas was much more than that with PEG [22,23]. For infants, glycerine
suppositories are to be used for disimpaction as enemas and lavage
solution are not indicated in them [16].
Maintenance therapy
To prevent re-accumulation after removing impaction
maintenance therapy in the form of dietary modification, toilet training
and laxatives needs to be started immediately after disimpaction or if
there is no impaction, then as a first step.
Dietary modification: The diet of
most children with functional constipation lacks fiber. Many of them are
predominantly on milk with very little complementary food. The children
with functional constipation should be encouraged to take more fluids,
absorbable and non-absorbable carbohydrate as a method to soften stools.
Non-absorbable carbohydrate (sorbitol) is found in some fruit juices
like apple, pear and prune juices. A balanced diet that includes whole
grains, fruits and vegetables is advised. The recommended daily fiber
intake is age (in years) + 5 in g/day. In our practice, where most
children are predominantly on milk diet, we counsel the parents to
restrict milk so that the child starts eating solid foods. Though cow
milk protein allergy (CMPA) was proposed as one the common causes of
constipation [24], subsequent studies [16,25] and our experience did not
substantiate that claim.
Toilet training: It should be imparted after 2 to
3 years of age. Too early and vigorous toilet training may be
detrimental for the child. The child is encouraged to sit on the toilet
for 5 to 10 minutes, 3 to 4 times a day immediately after major meals
for initial months [26]. The gastro-colic reflex, which goes into effect
shortly after a meal, should be used to advantage [27]. Children are
encouraged to maintain a daily record (stool diary) of bowel
movements, fecal soiling, pain or discomfort, consistency of stool and
the laxative dose. This helps to monitor compliance and to make
appropriate adjustment in the treatment program. Parents are instructed
to follow a reward system. Children should be rewarded for not soiling
and for regular sitting on the toilet. This acts as a positive
reinforcement for the child.
Laxatives: Table II [28] presents
the doses and side effects of various laxatives. It has been shown that
lactulose, sorbitol, milk of magnesia (magnesium hydroxide), and mineral
oil (castor oil), all are equally effective in children. Milk of
magnesia and mineral oil are unpalatable and due to the risk of lipoid
pneumonia mineral oil is contraindicated in infants. The commonly used
laxative in children so far was lactulose, until the introduction of
PEG. The study by Loening-Baucke [26] has shown that low volume (0.5 to
1g/kg/day) polyethylene glycol (PEG) without electrolytes is as
effective as milk of magnesia in the long-term treatment of constipation
in children. Low volume PEG has been compared with lactulose in the
treatment of childhood functional constipation and a meta-analysis of
five RCTs comprising of 519 children has shown that PEG was more
effective than lactulose with equal tolerability and fewer side effects
[29]. Side effects, especially bloating and pain are less with PEG. With
long term use, lactulose loses its efficacy due to change in gut flora
but PEG does not [30]. The dose of laxative should be adjusted to have
one or two soft stools/day without any pain or soiling. Once this target
is achieved, the same dose should be continued for at least 3 months to
help the distended bowel to regain its function. Point to be remembered
here is that laxative needs to be continued for several months and
sometimes years at the right dose. Early and rapid withdrawal is the
commonest cause for recurrence. Stimulant laxatives (senna, bisacodyl)
are not used routinely and are contraindicated in infants. They may be
used for a short course in refractory cases as a rescue therapy [16].
TABLE II Laxatives–dosage and Side Effects (Modified from NASPGHAN Position Statement) [28]
|
Drugs |
Dose |
Side effects |
|
Lactulose |
1-2 g/kg, 1-2 doses |
Bloating, abdominal cramps |
|
Sorbitol |
1-3 mL/kg/d, 1-2 doses |
Same as lactulose |
|
Milk of magnesia |
1-3 mL/kg/d, 1-2 doses |
Excess use leads to hypocalcemia, hypermagnesemia,
hypophosphatemia |
|
PEG for disimpaction |
25 mL/kg/hour (R/T) or 1-1.5 g/kg for 3-6 d |
Nausea, bloating, cramps, vomiting
|
|
PEG for maintenance |
5-10 mL/kg/d or 0.4 to 0.8 g/kg/d |
Nausea, bloating, cramps, vomiting |
|
Mineral oil for disimpaction |
15-30 mL/y of age (max. 240mL) |
Lipoid pneumonia, interference with absorption of fat soluble
vitamins |
|
Mineral oil for maintenance |
1-3 mL/kg/d
|
Lipoid pneumonia, interference with absorption of fat soluble
vitamins |
|
Senna |
2-6 yrs: 2.5-7.5 mL/day (8.8 mg/5mL) |
Melanosis coli, hepatitis, hypertrophic
|
|
6-12 yrs: 5-15 mL/d
|
osteoarthropathy, neuropathy |
|
Bisacodyl |
0.5-1 suppository (10 mg)1-3 tabs /dose(5mg) |
Abdominal pain, diarrhea, hypokalemia |
|
PEG: Polyethylene glycol; R/T: Ryle’s tube |
Follow-up schedule
A close and regular follow-up is a key to the success
of treatment of functional constipation. Initial follow-up should be
monthly till a regular bowel movement is achieved. After that it should
be 3 monthly for 2 years and then yearly [26]. On each visit, by
reviewing stool records and repeating abdominal and (if required) rectal
examination, progress should be assessed. If necessary, dosage
adjustment is to be made. Once a regular bowel habit is established, the
laxative dosage is to be decreased gradually before stopping.
Outcome
In a long-term follow up study (mean 6.9 ±2.7 years)
on 90 children, who were <4 years at diagnosis, Loening-Baucke [31]
showed that 63% had recovery but symptoms of chronic constipation
persisted in one third of cases 3 to 12 years after initial evaluation
and treatment. In another study, it has been shown that 50% of patients
were off laxative at 1 year, another 20% at 2 years and the remaining
30% were on laxative for many years [14]. von Ginkel, et al. [32]
in a long-term follow up (mean 5 years) study on 418 cases have also
shown that 60% were successfully treated at one year but 30% of cases in
the 16 years or older age group continued to have constipation. They
found that age at onset of constipation (<4 years) and associated fecal
incontinence were poor prognostic factors. In a large study on 300
children, Clayden [33] has shown that 22% required laxative for <6
months, 44% for <12 months and 56% for >12 months. By summarizing all
these studies it can be said that half to two thirds of children with
functional constipation had successful outcome with laxative therapy for
6 to 12 months but the remaining one thirds require long-term therapy
and they may continue to have constipation as an adult. Recurrence of
constipation after initial recovery is common (50% may have relapse
within a year of stopping therapy) but they respond well to retreatment
[12]. Poor prognostic factors are; early onset (<4 years), associated
with fecal incontinence, and longer duration of symptoms (>6months)
[16].
Refractory Constipation
A case of constipation is labeled as refractory when
there is no response to optimal conventional treatment for at least 3
months [16]. The prevalence of refractory constipation is said to be
20-30% [16, 34] but the prevalence is much higher in India at primary
care pediatrician level due to lack of awareness about optimal
conventional treatment. At primary care level, disimpaction is hardly
practiced and as a result of which the response of laxative therapy is
not optimal. The second important reason is early discontinuation of
therapy which leads to refractoriness of constipation. The true
refractory constipation is extremely uncommon in primary care set up.
Even at tertiary care centers, refractory constipation is uncommon [2].
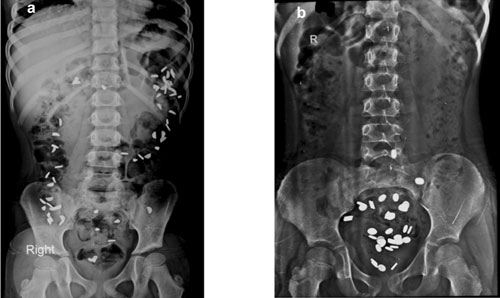 |
|
Fig. 4 (a) Colon transit time (CTT)
study by radio-opaque markers showing slow transit constipation;
4(b) Functional outlet obstruction.
|
Besides organic causes of constipation, motility
disorders (like slow transit constipation), disorders of stool expulsion
like dyssynergic defecation, internal anal sphincter achalasia and
sphincter dysfunction in children with Hirschsprung disease which
persist after surgery are important causes of refractory constipation
[34]. While approaching refractory constipation common organic causes (Fig.2)
like Hirschsprung disease, hypothyroidism, celiac disease, hypercalcemia,
spinal cord abnormalities should be ruled out first and then motility
studies (like colon transit time [CTT], anorectal manometry with balloon
expulsion test, colonic manometry) to be done to find out motility
disorders [34, 35]. The simplest and the most informative of all these
tests is colon transit time (CTT) study which can be done by
radio-opaque markers and by radionuclide scintigraphy (NTS or nuclear
transit studies) [34]. In radiographic CTT study, a capsule containing
20 radio-opaque markers (different shape in different days) are given
daily for 3 days and plain x-ray abdomen is taken on day four and if
required on day 7 (when all markers are retained on day 4). From X-ray,
markers are counted in right colon, left colon and recto-sigmoid regions
and the mean segmental time is calculated. Slow transit constipation is
defined as retention of markers for 62 hours or more [36, 37]. As per
the CTT study, constipation can be divided into three categories; (i)
normal transit constipation, (ii) functional outlet obstruction
or dyssynergic defecation (retention of markers in rectosigmoid region)
and (iii) slow transit constipation (retained markers are
distributed all over) (Fig. 4a and 4b).
In a study of 225 children (135 pediatric constipation, 56 non-retentive
fecal incontinence and 24 recurrent abdominal pain) Benninga, et al.
[36,37] have shown that 56% of constipated children had normal CTT, 24%
had functional outlet obstruction and just 20% had slow transit
constipation. In another study on 85 children with functional
constipation with rectal fecal impaction by Bekkali, et al. [20]
have shown that 93% had delayed CTT and as expected majority (83.5%) of
them had delayed rectosigmoid segment CTT. As the basic pathophysiology
of functional constipation is voluntary withholding of feces, it is
expected that most children with functional constipation will have
either functional outlet obstruction/dyssynergic defecation or normal
transit constipation.
In normal defecation there is synchronized relaxation
of puborectalis muscle (makes ano-rectal angle straight) and external
anal sphincter along with generation of propulsive force through
contraction of colon and increased in intra-abdominal pressure, which
propels stools out of rectum. In dyssynergic defecation there is
paradoxical contraction or failure of relaxation of external anal
sphincter and puborectalis muscle with or without increased rectal
pressure (propulsive force) [38]. These features are detected on
anorectal manometry. Therapeutic option of refractory constipation due
to dyssynergic defecation is biofeedback (to restore the normal pattern
of defecation) and for slow transit constipation is to enhance colonic
transit with newer drugs like colon-specific prokinetics like
prucalopride (5HT4 agonist) [39] and intestinal secretagogue (lubiprostone)
[40], which increases intestinal chloride secretion and accelerates
small intestinal and colonic transit. Antegrade continence enema helps
in refractory slow transit constipation cases [41].
|
BOX 2
Clinical Features of Slow Transit Constipation in Children [42] |
|
• High frequency of delayed passage of meconium
• Onset of symptoms early in first year
and/or failure to toilet training
• Feces soft rather than rock hard
• Failure of high fiber diets (they tend to
make symptoms worse)
• Global delay in colonic transit on transit study.
|
Most reports of slow transit constipation in children
are from Australia and the clinical presentations of this subset of
patients are different from functional constipation (Box 2).
In a study of 100 children with slow transit constipation, Hutson, et
al. [42,43] have shown that a history of delayed passage of meconium
was seen in 30% of cases, onset of severe constipation in infancy in 63%
and half (52%) of those presenting after 2 years of age had history of
soiling (fecal incontinence) and failure of toilet training, and the
majority (90%) had no hard fecal mass in rectosigmoid area. The
management of slow transit constipation is quite difficult as they do
not respond to conventional laxative therapy and the main concern is
soiling. Fiber therapy is contraindicated (as the motility is slow), the
newer drugs like colon specific prokinetics like prucalopride [39] and
chloride channel activator (lubiprostone) [40] are still investigational
drugs in children. The only effective therapy for this subset of
patients is antegrade continence enema. Here, appendix is used as
conduit to insert cecostomy button (Chait trapdoor button) to give enema
[44,45]. It has minimal scar and just a button at right iliac fossa
which is used in the morning to give antegrade enema and the whole day
patient remains dry (no soiling). In a recent study on 203 cases (median
age 10 years, follow up 5.5 years, 62% due to refractory chronic
idiopathic constipation) of this modality, Randall, et al. [41]
showed good result in 93%, soiling prevented in 75% and symptoms
resolved (no longer on antegrade continence enema) in 26% (81% of them
were chronic idiopathic constipation).
Colonic manometry plays an important role in guiding
both medical and surgical treatment in refractory constipation. In fact
it has been shown that the success of antegrade continence enema
procedure depends on colonic manometry results [46]. If there is
generalized colonic dysmotility (absence of high-amplitude propagating
contraction [HAPC] in the entire colon) then there is no point in
putting cecostomy catheter. Similarly, colonic manometry results can
dictate the type of surgery following colonic diversion; subtotal
colectomy if small bowel motility is normal but whole colonic motility
is abnormal, left hemicolectomy if only left colonic motility is
abnormal and reanastomosis if colonic motility is normal [47].
A relatively less common but important cause of
refractory constipation is internal anal sphincter achalasia. In a study
of 332 patients with severe constipation, De Caluwe, et al. [48]
have reported this as a cause in just 4.5% of cases. This subset of
patients usually present with severe constipation (99.7%) which often
associated with fecal incontinence (46%) and are diagnosed by absence of
anorectal inhibitory reflex (ARIR) on anorectal manometry along with
presence of ganglion cell on rectal biopsy [49]. The treatment options
for internal anal sphincter achalasia are posterior anal sphincter
myectomy and intrasphincteric botulinum toxin injection. In a recent
meta-analysis, it has been shown that former is better [49].
Conclusions
Constipation is quite common in Asia, and most often
of functional origin. Detailed history and proper physical examination,
including digital rectal examination, can easily differentiate
functional from organic constipation. There is no need to do any
investigation before starting treatment in functional constipation.
Disimpaction with oral polyethylene glycol is the main step in the
management and skipping this step leads to refractoriness of
constipation. Polyethylene glycol is shown to be superior to lactulose
in the management of constipation. In most cases, prolonged (months to
years) laxative therapy is required and early withdrawal leads to
recurrence. Radiological colon transit time study plays an important
role in the management of refractory constipation. Slow transit
constipation is altogether a different entity and antegrade continence
enema helps in this subset of patients.
Funding: None; Competing interests: None
stated.
References
1. Van den Berg MM, Benninga MA, Di Lorenzo C.
Epidemiology of childhood constipation: a systematic review. Am J
Gastroenterol 2006;101:2401-9.
2. Khanna V, Poddar U, Yachha SK. Constipation in
Indian children: need for knowledge not the knife. Indian Pediatr.
2010;47:1025-30.
3. Rajindrajith S, Devanaryana NM, Adhikari C,
Pannala W, Benninga MA. Constipation in children: an epidemiological
study in Sri Lanka using Rome III criteria. Arch Dis Child.
2012;97:43-5.
4. Aziz S, Fakih HAM, Di Lorenzo C. Bowel habits and
toilet training in rural and urban dwelling children in a developing
country. J Pediatr. 2011;158:784-8.
5. Steer CD, Emond AM, Golding J, Sandhu B. The
variation in stool patterns from 1 to 42 months: a population bases
observational study. Arch Dis Child. 2009;94:231-4.
6. den Hertog J, van Leengoed E, Kolk F, van den
Broek L, Kramer E, Bakker E, et al. The defecation pattern of
healthy term infants up to the age of 3 months. Arch Dis Child Fetal
Neonatal Ed. 2012;97:F465-F470.
7. Tunc VT, Camurdan AD, Ilhan MN, Sahin F, Beyazova
U. Factors associated with defecation patterns in 0 to 24 months old
children. Eur J Pediatr. 2008;167:1357-62.
8. Hyman PE, Milla PJ, Benninga MA, Davidson GP,
Fleisher DF, Taminiau J. Childhood functional gastrointestinal
disorders: neonate/ toddler. Gastroenterology. 2006;130:1519-26.
9. Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E,
Hyams JS, Staiano A. Childhood functional gastrointestinal disorders:
child/adolescents. Gastroenterol. 2006; 130:1527-37.
10. Levine MD. Children with encopresis: a
descriptive analysis. Pediatrics. 1975;56:412-6.
11. Taitz LS, Water JKH, Urwin OM, Molnar D. Factors
associated with outcome in management of defecation disorders. Arch Dis
Child. 1986;61:472-7.
12. Amendola S, De-Angelis P, Dall’Oglio L, Di
Abriola GF, Di Lorenzo M. Combined approach to functional constipation
in children. J Pediatr Surg. 2003;38:819-23.
13. Loening-Baucke V. Constipation in early
childhood: Patient characteristics, treatment and long-term follow up.
Gut. 1993;34:1400-4.
14. Loening-Baucke V. Chronic constipation in
children. Gastroenterol. 1993;105:1557-64.
15. Partin JC, Hamill SK, Fischel JE, Partin JS.
Painful defecation and fecal soiling in children. Pediatrics.
1992;89:1007-9.
16. Tabbers MM, Di Lorenzo C, Berger MY, Faure C,
Langendam MW, Nurko S, et al. Evaluation and treatment of
functional constipation in infants and children: evidence-based
recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr.
2014;58:258-74.
17. Metaj M, Laroia N, Lawrence RA, Ryan RM.
Comparison of breast- and formula-fed normal new born in time to first
stool and urine. J Perinatol. 2003;23 624-8.
18. Jung PM. Hirschsprung’s disease: one surgeon’s
experience in one institution. J Pediatr Surg. 1995;30:646-51.
19. Loening-Baucke V. Factors determining outcome in
children with chronic constipation and fecal soiling. Gut. 1989;30
999-1006.
20. Bekkali N, van den Berg MM, Dijkgraaf MGW, van
Wijk MP, Bongers MEJ, Liem D, et al. Rectal fecal impaction
treatment in childhood constipation: enemas versus high doses oral PEG.
Pediatric. 2009;124:e1108-e15.
21. Youssef NN, Peters JM, Henderson W, Shultz-Peters
S, Lockhart DK, Di Lorenzo C. Dose response of PEG 3350 for the
treatment of childhood fecal impaction. J Pediatr. 2002;141: 410-4.
22. Guest JF, Candy DC, Clegg JP, Edwards D, Helter
MT, Dale AK, et al. Clinical and economical impact of using
macrogol 3350 plus electrolytes in an outpatient setting compared to
enemas and suppositories and manual evacuation to treat pediatric fecal
impaction based on actual clinical practice in England and Wales. Curr
Med Res Opin. 2007;23:2213-25.
23. Freedman SB, Thull-Freedman J, Rumantir M,
Eltorki M, Schuh S. Pediatric constipation in the emergency department:
evaluation, treatment and outcomes. J Pediatr Gastroenterol Nutr.
2014;59:327-33.
24. Iacono G, Cavataio F, Montalto G, Florena A,
Tumminello M, Soresi M, et al. Intolerance of cow’s milk and
chronic constipation in children. N Engl J Med. 1998;339:1100-4.
25. Simeone D, Miele E, Boccia G, Marino A, Troncone
R, Staiano A. Prevalence of atopy in children with chronic constipation.
Arch Dis Child. 2008;93:1044-7.
26. Loening-Baucke V. Polyethylene glycol without
electrolytes for children with constipation and encopresis. J Pediatr
Gastroenterol Nutr. 2002;34:372-7.
27. Lowery SP, Srour JW, Whitehead WE, Schuster MM.
Habit training as treatment of encopresis secondary to chronic
constipation. J Pediatr Gastroenterol Nutr. 1985;4:397-401.
28. Baker SS, Liptak GS, Colletti RB, Croffie JM, Di
Lorenzo C, Ector W, et al. Clinical practice guideline:
Evaluation and treatment of constipation in infants and children:
recommendations of the North American Society of Pediatric
Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr.
2006;43:e1-e13.
29. Candy D, Belsey J. Macrogol (polyethylene glycol)
laxatives in children with functional constipation and fecal impaction:
a systematic review. Arch Dis Child. 2009;94:156-60.
30. Candelli M, Nista EC, Zocco MA, Gasbarrini A.
Idiopathic chronic constipation; pathophysiology, diagnosis and
treatment. Hepatogastroenterol. 2001;48:1050-7.
31. Loening-Baucke V. Constipation in early
childhood: patient characteristics, treatment and long-term follow-up.
Gut. 1993;34:1400-4.
32. van Ginkel R, Reitsma JB, Buller HA, van Wijk MP,
Taminiau JA, Benninga MA. Childhood constipation: longitudinal follow-up
beyond puberty. Gastroenterology 2003;125:357-63.
33. Clayden GS. Management of chronic constipation.
Arch Dis Child. 1992;67:340-4.
34. Southwell BR, King SK, Hutson JM. Chronic
constipation in children: organic disorders are a major cause. J Pediatr
Child Health. 2005;41:1-15.
35. Kwshtgar A, Ward HC, Clayden GS. Diagnosis and
management of children with intractable constipation. Semin Pediatr
Surg. 2004;13:300-9.
36. Benninga MA, Voskuijl WP, Akkerhuis GW, Taminiau
JA, Buller HA. Colonic transit times and behavior profiles in children
with defecation disorders. Arch Dis Child. 2004;89: 13-6.
37. Benninga MA, Buller HA, Staalman CR, Gubler FM,
Bossuyt PM, van der Plas RN, et al. Defecation disorders in
children, colonic transit times versus the Barr-score. Eur J Pediatr.
1995;154:277-84.
38. Rao SS. Dyssynergic defecation and biofeedback
therapy. Gastroenterol Clin North Am. 2008;37:569-86.
39. Winter HS, Di Lorenzo C, Benninga MA, Gilger MA,
Kearns GL, Hyman PE, et al. Oral prucalopride in children with
functional constipation. J Pediatr Gastroenterol Nutr. 2013;57:197-203.
40. Hyman PE, Di Lorenzo C, Prestridge LL, Youssef
NN, Ueno R. Lubiprostone for the treatment of functional constipation in
children. J Pediatr Gastroenterol Nutr. 2014;58:283-91.
41. Randall J, Coyne P, Jaffray B. Follow up of
children undergoing antegrade continent enema: experience of over two
hundred cases. J Pediatr Surg. 2014;49:1405-8.
42. Hutson JM, McNamara J, Gibb S, Shin YM. Slow
transit constipation in children. J Pediatr Child Health.
2001;37:426-30.
43. Wheatley JM, Hutson JM, Chow CW, Oliver M, Hurley
MR. Slow transit constipation in childhood. J Pediatr Surg.
1999;34:829-33.
44. Malone PS, Ransley PG, Kiely EM. Preliminary
report: the antegrade continence enema. Lancet. 1990;336:1217-8.
45. Chait PG, Shandling B, Richards HF. The cecostomy
button. J Pediatr Surg. 1997;32;849-51.
46. Van den Berg MM, Hogan M, Caniano DA, Di Lorenzo
C, Benninga MA, Mousa HM. Colonic manometry as predictor of cecostomy
success in children with defecation disorders. J Pediatr Surg.
2006;41:730-6.
47. Villarreal J, Sood M, Zangen T, Flores A, Michel
R, Reddy N, et al. Colonic diversion for intractable constipation
in children: colonic manometry helps guide clinical decisions. J Pediatr
Gastroenterol Nutr. 2001;33:588-91.
48. De Caluwe D, Yoneda A, Akl U, Puri P. Internal
anal sphincter achalasia: outcome after internal sphincter myectomy. J
Pediatr Surg. 2001;36:736-8.
49. Florian F, Puri P. Comparison of posterior
internal anal sphincter myectomy and intrasphincteric botulinum toxin
injection for treatment of internal anal sphincter achalasia: A
meta-analysis. Pediatr Surg Int. 2012;28:765-71.
|
|
|
 |
|

