|
|
|
Indian Pediatr 2013;50:
394-398 |
 |
Triglyceride and Non-High-Density Lipoprotein
Cholesterol as Predictors of Cardiovascular Disease Risk Factors
in Chinese Han Children
|
|
Wei Fen Zhu, Li Liang, *Chun Lin Wang and Jun Fen Fu
From the Department of Endocrinology, Children’s
Hospital of Zhejiang University School of Medicine; and *Key Laboratory
of Reproductive Genetics (Zhejiang University), Ministry of Education;
Hangzhou, China.
Correspondence to: Prof Li Liang, Department of
Endocrinology, Children’s Hospital of Zhejiang University School of
Medicine. 57 Zhugan Xiang, Hangzhou 310003, China.
Email: [email protected]
Received: May 31, 2012;
Accepted: September 24, 2012.
Published online: 2012, October 5.
PII: S097475591200455
|
|
Objective: To investigate the role of serum cholesterol and
triglyceride in the assessment of cardiovascular disease risk factors in
children and adolescents.
Design: Case-control study.
Setting: Children’s Hospital of
Zhejiang University School of Medicine, Hangzhou, China.
Subjects: Children from 6 years
to 17 year old. 188 with simple obesity, and 431 with obesity and
metabolic abnormalities. 274 age and gender-matched healthy children as
controls.
Methods: Receiver operating
characteristic curves were used to analyze the detection of
cardiovascular disease risk factors by cholesterol and triglyceride in
children and adolescents.
Results: The ranges of areas
under receiver operating characteristic curves (AUC) for triglyceride
and non-high-density lipoprotein cholesterol were 0.798-0.860 and
0.667-0.749, respectively to detect cardiovascular disease risk factors.
The ranges of AUC for low-density lipoprotein cholesterol, total
cholesterol, and high-density lipoprotein cholesterol were 0.631-0.718,
0.596-0.683, and 0.292-0.376, respectively.
Conclusions: Triglyceride and
non-high-density lipoprotein cholesterol are better than low-density
lipoprotein cholesterol as predictors of cardiovascular disease risk
factors in Chinese Han children and adolescents.
Key words: Cardiovascular disease, Children,
Cholesterol, Lipids, Risk factors, Triglyceride.
|
|
A
lthough atherosclerosis manifests clinically in
middle and late adulthood, it is known to have a long asymptomatic phase
of development, that begins early in life, often during childhood, and
is significantly related to dyslipidemias. Dyslipidemia, characterised
by elevated total cholesterol (TC), low-density lipoprotein cholesterol
(LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C) and
triglyceride (TG) levels as well as low high-density lipoprotein
cholesterol (HDL-C) concentration, is well-known cardiovascular disease
(CVD) risk factor [1].
With respect to lipid profiling for CVD risk
assessment, LDL-C levels are widely targeted for primary prevention and
intervention. At present, however, some investigators have suggested
that non-HDL cholesterol may be superior to LDL cholesterol alone as a
predictor of CVD risk factors in adolescents [2] and adults [3], largely
because cholesterol-enriched very-low-density lipoprotein and
intermediate-density lipoprotein have been shown to be atherogenic in
addition to LDL. As for TG, the relationship between TG and CVD risk
factors is controversial. In some studies, the relationship is not
statistically significant after controlling for other lipids,
particularly HDL-C [4-6]. However, several meta-analyses have concluded
that TG is a CVD risk factor independent of HDL-C and other risk factors
[7-9].
As clusters of risk factors for CVD are stable
characteristics that tend to track fairly well from childhood into
adulthood [10], preventive efforts that start in childhood are
necessary, as they could delay progression to clinical disease. It is,
thus important is to identify early the cardiovascular risk factors in
children and adolescents. The associations between lipid parameters and
CVD risk factors has been described in population groups [2,11] but
similar studies in children and adolescents in China are relatively
sparse. We therefore conducted this study to compare the predictive
value of serum cholesterol and triglyceride in CVD risk factors in
children and adolescents.
Methods
Children and adolescents between 6 and 17 years of
age (n=619) who were referred to our endocrinology department
between September 2008 to September 2010 with the complaint of obesity
were enrolled in this study. Subjects were eligible if they were healthy
and had a body-mass index (BMI) that exceeded the 95th percentile for
their age and sex [12]. According to the presence or absence of
metabolic abnormalities, the total 619 cases were divided into the
Simple obesity group and the Obesity with metabolic abnormalities group.
The control group consisted of 274 healthy children and adolescents, all
of whom were recruited by the Department of Child Care for health
examination. Exclusion criteria consisted of the known presence of
diabetes or other endocrine metabolic or kidney diseases, and the use of
medication that alters blood pressure, glucose, or lipid metabolism.
Consent was obtained from the parents and the Ethics
committee of the Children Hospital of Zhejiang University School of
Medicine.
The participants were classified as having metabolic
abnormalities if they met one or more of the following criteria for age
and sex: elevated systolic blood pressure (SBP) or diastolic blood
pressure (DBP) (a value that exceeded 95 th
percentile for age and sex) [13], abnormal fasting blood glucose (FBG)
(glucose level >126 mg/dL) [14]; and dyslipidemia. The diagnosis of
dyslipidemia was achieved if any of the following was found: a TC level
>5.18mmol/L; a TG level >1.47mmol/L; an LDL-C level >3.37mmol/L; a
non-HDL-C level >3.76 mmol/L or a HDL-C level <1.03 mmol/L [15,16].
Body height was measured to the nearest 1 mm, with
the participants in bare or stocking feet standing upright against a
stadiometer. With the participants lightly dressed, bodyweight was
measured to the nearest 0.1 kg by a medical digital scale. Waist
circumference (WC) was measured to the nearest 1 mm by placing a tape
measure around participant’s body in the horizontal plane, at the level
of the midpoint between the lowest rib and the iliac crest on bare skin
when in a state of expiration. We used the standard hydrargyric cuff
sphygmomanometer for blood pressure measurement. The measurements were
done by practitioners who received professional training. Every
participant was seated and in a relaxed state for at least 10 min before
measurement. Each underwent blood pressure measurement three times, the
gap between the highest and lowest value was below 4 mmHg, the average
value of the three values was used, or another measurement was made
after the subject had rested.
Baseline blood samples were obtained from subjects at
8 A.M., after a 10-hour overnight fast, with the use of an indwelling
venous line for measurement of levels of glucose and lipids (TC, TG,
LDL-C, HDL-C). Blood glucose was measured using a glucose oxidase
method. The concentrations of serum TC and TG were detected by the
routine enzymatic method. Plasma HDL-C and LDL-C concentration were
determined by the direct measurement method.
BMI was calculated as body weight/(body height) 2 ,
waist-to-height radio (WHtR) was calculated as WC/height, and non-HDL-C
was calculated as total cholesterol minus HDL-C. Age- and sex-specific
BMI Z-scores, WC Z-scores, and WHR Z-scores were
used as continuous dependant variables for each model [17].
Statistics analysis: Statistical analyses
were conducted using SPSS software (version 17.0). Quantitative data
with normal distributions were presented as mean ± SD. Chi-square test
was used to compare proportions between the groups. Continuous variables
were analyzed with Student’s t test. Differences were considered
statistically significant if P<0.05. Receiver operating
characteristic (ROC) curves were used to analyze the detection of
cardiovascular risk factors by cholesterol and triglyceride in children
and adolescents.
Results
The descriptive characteristics of the sample are
presented in Table I. Children in Group 3 exhibited higher
BMI; BMI Z-scores; WC and WC Z-scores than the ones in
Group 2.
TABLE I Baseline Characteristics of the Study Population
|
Control Group
(Group 1) |
Simple Obesity Group
(Group 2) |
Obesity with Metabolic
Abnormalities
|
|
|
|
Group (Group 3) |
|
Gender (M/F) |
191/83 |
147/41 |
311/120 |
|
Age (y) |
10.29 ± 2.82 |
10.35 ± 1.82 |
10.67 ± 2.12 |
|
BMI (kg/m2) |
16.79 ± 1.83 |
27.00 ± 3.63** |
27.87 ± 3.58 ** ## |
|
BMI Z-scores |
(-0.20 ± 0.52) |
3.06 ± 1.21** |
3.32 ± 1.46**#
|
|
WC (cm)
|
57.88 ± 7.41 |
85.08 ± 10.80** |
88.51 ± 11.41**## |
|
WC Z-scores |
(-0.38) ± 0.53 |
2.36 ± 1.61** |
2.84 ± 1.32**## |
|
WHtR |
0.41 ± 0.03 |
0.59 ± 0.11** |
0.60 ± 0.08 ** |
|
WHtR Z-scores |
(-0.32) ± 0.57 |
2.78 ± 2.75** |
3.04 ± 1.41** |
|
BMI, Body mass index; WC, waist circumstance; WHtR,
waist-to-height; Compared to Group 1 *represents P<0.05,
** represents P<0.01, Compared to Group 2 #represents P<0.05,
##represents P<0.01.
|
There was a trend of increased cardiovascular risk in
obese children. SBP; DBP; FBG; TG; LDL-C and non-HDL-C increased
stepwise, whereas HDL-C decreased stepwise in Group 1, Group 2 and Group
3. Compared to Group 1 and Group 2, Group 3 had significantly higher TC
levels, while no significant difference was apparent in TC level between
Group 1 and Group 2 (Table II).
TABLE II Cardiovascular Risk Factors of the Study Population
|
Control Group
|
Simple Obesity Group |
Obesity with Metabolic
|
|
(Group 1) |
(Group 2) |
Abnormalities Group (Group
3) |
|
SBP (mmHg) |
91.04 ± 11.09 |
108.10 ± 11.21** |
114.12 ± 13.88**## |
|
DBP (mmHg) |
64.83 ± 7.44 |
66.99 ± 7.39** |
69.13 ± 8.99**## |
|
FBG (mmol/L) |
4.83 ± 0.47 |
4.91 ± 0.33* |
5.13 ± 0.75**## |
|
TC (mmol/L) |
3.97 ± 0.58 |
4.05 ± 0.62 |
4.50 ± 0.95**## |
|
TG (mmol/L) |
0.75 ± 0.28 |
0.95 ± 0.28** |
1.70 ± 0.89**## |
|
HDL-C (mmol/L) |
1.49 ± 0.28 |
1.36 ± 0.34** |
1.21 ± 0.31**## |
|
LDL-C (mmol/L) |
2.09 ± 0.52 |
2.25 ± 0.51** |
2.67 ± 0.74**## |
|
non-HDL-C (mmol/L) |
2.48 ± 0.55 |
2.69 ± 0.60** |
3.29 ± 0.89**## |
|
SBP, systolic blood pressure; DBP,
diastolic blood pressure; FBG, fasting blood glucose; TC, total
cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein
cholesterol; LDL-C, low-density lipoprotein cholesterol;
non-HDL-C, non-high-density lipoprotein cholesterol. Compared to
Group 1*represents P<0.05, **represents P<0.01, Compared to
Group 2³#represents P<0.05, ##represents P<0.01. |
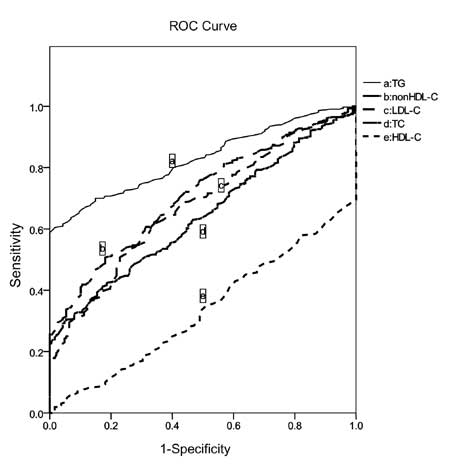
Fig.1 displays the areas under the curves
(AUC) for each lipid parameter as a predictor of cardiovascular risk
factors and comparisons of the AUC between Group 2 and Group 3. For the
identification of CVD risk, AUC for TG, non-HDL-C, LDL-C and TC were
0.829 (95% confidence interval [CI]: 0.798-0.860), 0.708 (95% CI:
0.667-0.749), 0.675 (95% CI: 0.631-0.718), 0.639 (95% CI:0.596-0.683),
respectively. These AUC were significantly greater than 0.5, in
detecting ardiovascular risk factors as compared to HDL-C with an area
of 0.334 (95% CI: 0.292-0.376). All plasma lipid parameters performed
significantly better among those in the Group 3 cohort than the Group 2
cohort (P<0.05).
 |
|
Fig. 1 Receiver operating
characteristic curves (ROC) constructed using the lipid
parameters.
TC, total cholesterol; TG, triglycerides;
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density
lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein
cholesterol.
|
Discussion
Our study shows a strong correlation between
childhood obesity and early-onset dyslipidemia, hypertension, and
hyperglycemia. These conditions, when manifested in childhood, track
into in adult life because obese children are more likely to become
obese adults [18].
We plotted the ROC curves for serum cholesterol and
triglycerides. The area under the ROC curve was largest for TG,
indicating the model was superior to the other CVD risk-prediction
models. In the past, the role of elevated triglycerides as an
independent CVD risk factor has been debated. However, emerging evidence
points to elevated triglyceride levels as a risk factor for
cardiovascular disease that is independent of HDL cholesterol levels.
One recent study consisting of 909 public parochial suburban
schoolchildren aged 6 to 18 years, found that over a follow-up of 26
years, adult CVD was associated with pediatric high TG (odds ratio [OR],
5.85; 95% CI, 2.3-14.7) by stepwise logistic regression [19], supporting
the hypothesis that TG would be an independent risk factor for CVD. A
meta-analysis of prospective studies has shown that for each 1 mmol/L
increase in TG, CVD risk increases by 12% in men and 37% in women,
irrespective of HDL-C and other risk factors [20]. Since an area under
the curve above 0.7 indicates a reasonably good clinical test,
monitoring the levels of TG among children and adolescents at increased
risk of obesity may be clinically useful in detecting their CVD risk
factors.
Recent studies involving subjects of different age
groups have also shown the importance of non-HDL-C as a reliable, less
costly parameter that is strongly correlated with cardiovascular risk
because non-HDL-C includes all atherogenic lipid subfractions [21]. Data
from the Bogalusa Heart Study suggest that childhood nonHDLcholesterol
levels persist and best predict adult dyslipidemia and other CVD risks
[22]. Another recent study, using data from the Framingham Heart Study,
showed that non-HDL-C was a better predictor of cardiovascular disease
risk than LDL-C [23]. These findings are consistent with the findings of
the present study, in which non-HDL-C was shown to outperform LDL-C.
Another major advantage of non-HDL-C is that it can be accurately
calculated in a non-fasting state and is therefore very practical to
obtain in clinical practice. In 2011, the Expert Panel on Integrated
Guidelines for Cardiovascular Health and Risk Reduction in Children and
Adolescents released its summary report, which recommends non-HDL-C as a
predictor of CVD risk [15]. In addition, non-HDL-C is included in the
diagnostic criteria of metabolic syndrome by Chinese Society of
Pediatrics [16].
Given the unrelenting rise in childhood obesity
rates, we have to brace ourselves for the onslaught of dyslipidemia and
other metabolic disorders in children and adolescents in the very near
future. Recently, the American Academy of Pediatrics issued a policy
statement on lipid screening and cardiovascular health in childhood
[24]. A fasting lipid profile is the recommended approach to screening,
because there is currently no noninvasive method to assess
atherosclerotic CVD in children and the first screening should
preferably take place after 2 years of age but no later than 10 years of
age. Our current study suggests that pediatric screening for lipid
parameters in children and adolescents has a crucial predictive value
for CVD risk factors, especially serum triglyceride and non-HDL-C.
Contributors: WZ: had primary responsibility for
patient screening, enrollment, outcome assessment, preliminary data
analysis and writing the manuscript. CW and JF: participated in the
development of the protocol and analytical framework for the study and
contributed to the writing of the manuscript. LL: supervised the design
and execution of the study and contributed to the writing of the
manuscript.
Funding: The
National Key Technology R&D Program of ChinaÿGrant No.2009BAI80B01, and
Zhejiang Science and Technology Agency (Grant No.2008C03002-1).
Competing interests: None stated.
|
What is Already Known?
• Role of dyslipidemia in the assessment of
cardiovascular disease risk factors in children and adolescents.
What This Study Adds?
• Triglyceride and non-high density
lipoprotein cholesterol are better than low-density lipoprotein
cholesterol as predictors of cardiovascular disease risk factors
in Chinese Han children and adolescents.
|
References
1. Jago R, Harrell JS, McMurray RG, Edelstein S, EI
Ghormli L, Bassin S. Prevalence of abnormal lipid and blood pressure
values among an ethnically diverse population of eighth-grade
adolescents and screening implications. Pediatrics. 2006;117:
2065-73.
2. Srinivasan SR, Frontini MG, Xu J, Berenson GS.
Utility of childhood non-high-density lipoprotein cholesterol levels in
predicting adult dyslipidemia and other cardiovascular risks:The
Bogalusa Heart Study. Pediatrics. 2006;118:201-6.
3. Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer
MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein
B in the prediction of coronary heart disease in men. Circulation.
2005;112:3375-83.
4. Austin MA. Epidemiologic associations between
hypertriglyceridemia and coronary heart disease. Semin Thromb Hemost.
1988;14:137-42.
5. Austin MA. Plasma triglyceride as a risk factor
for coronary heart disease. The epidemiologic evidence and beyond. Am J
Epidemiol. 1989;129:249-59.
6. Freedman DS, Gruchow HW, Anderson AJ, Rimm AA,
Barboriak JJ. Relation of triglyceride levels to coronary artery
disease: the Milwaukee Cardiovascular Data Registry. Am J Epidemiol.
1988;127:1118-30.
7. Abdel-Maksoud MF, Hokanson JE. The complex role of
triglycerides in cardiovascular disease. Semin Vasc Med. 2002;2:325-33.
8. Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H,
Whitlock G. Asia Pacific Cohort Studies Collaboration. Serum
triglycerides as a risk factor for cardiovascular diseases in the
Asia-Pacific region. Circulation. 2004;110:2678-86.
9. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G,
Wareham N, Bingham S. Triglycerides and the risk of coronary heart
disease: 10,158 incident cases among 262,525 participants in 29 Western
prospective studies. Circulation. 2007;115:450-8.
10. Bao W, Srinivasan SR, Wattigney WA, Berenson GS.
Persistence of multiple cardiovascular risk clustering related to
syndrome X from childhood to young adulthood. Arch Intern Med.
1994;154:1842-7.
11. Morrison JA, Glueck CJ, Horn PS, Yeramaneni S,
Wang P. Pediatric triglycerides predict cardiovascular disease events in
the fourth to fifth decade of life. Metabolism. 2009;58:1277-84.
12. Group of China Obesity Task Force. Body mass
index reference norm for screening overweight and obesity in Chinese
children and adolescents. China J Epidemiol. 2004;2:97-102.
13. National High Blood Pressure Education Program
Working Group on Hypertension Control in Children and Adolescents.
Update on the 1987 Task Force Report on High Blood Pressure in Children
and Adolescents: A Working Group Report From the National High Blood
Pressure Education Program. Pediatrics. 1996;98:649-58.
14. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink
M, Arslanian S. The metabolic syndrome in children and adolescents.
Lancet. 2007;369:2059-61.
15. Expert Panel on Integrated Guidelines for
Cardiovascular Health and Risk Reduction in Children and Adolescents;
National Heart, Lung, and Blood Institute. Expert panel on integrated
guidelines for cardiovascular health and risk reduction in children and
adolescents: summary report. Pediatrics. 2011;128: S213-56.
16. The Subspecialty Groups of Endocrinology and
Genetic Disease, Pediatric Cardiology and Child Health Care, The Society
of Pediatrics, Chinese Medical Association. Definition and prevention
recommendations of metabolic syndrome in children and adolescents.
China J Pediatr. 2012; 50.
17. Xue Feng Chen, Li Liang, Jun Fen Fu, Chun Xiu
Gong, Feng Xiong, Ge Li Liu, et al. Study on physique index set
for Chinese children and adolescents. Chlin J Epidemiol. 2012;
33:449-54.
18. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz
WH. Predicting obesity in young adulthood from childhood and parental
obesity. N Engl J Med. 1997;337:869-73.
19. Morrison JA, Glueck CJ, Wang P. Childhood risk
factors predict cardiovascular disease, impaired fasting glucose plus
type 2 diabetes mellitus, and high blood pressure 26 years later at a
mean age of 38 years: the Princeton–lipid research clinics follow-up
study. Metabolism. 2012;61:531-41.
20. Abdel-Maksoud MF, Hokanson JE. The complex role
of triglycerides in cardiovascular disease. Semin Vasc Med.
2002;2:325-33.
21. Srinivasan SR, Myers L, Berenson GS. Distribution
and correlates of non-high-density lipoprotein cholesterol in children:
the Bogalusa heart study. Pediatrics. 2002;1103:e29.
22. Freedman DS, Khan LK, Dietz WH, Srinivasan SR,
Berenson GS. Relationship of childhood obesity to coronary heart disease
risk factors in adulthood: the Bogalusa Heart Study. Pediatrics.
2001;108:712-8.
23 . Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan
M, Grundy SM. Non-high-density lipoprotein and very-low-density
lipoprotein cholesterol and their risk predictive values in coronary
heart disease. Am J Cardiol. 2006;98:1363-8.
24. Daniels SR, Greer FR, Committee on Nutrition.
Lipid screening and cardiovascular health in childhood. Pediatrics.
2008;122:198-208.
|
 |
|

