|
|
|
Indian Pediatr 2013;50:
390-393 |
 |
Oral Zinc Supplementation for Reducing
Mortality in Probable Neonatal Sepsis: A Double Blind
Randomized Placebo Controlled Trial
|
|
K Mehta, NK Bhatta, *S Majhi, MK Shrivastava and RR Singh
From the Departments of Pediatrics and Adolescent Medicine and
*Biochemistry, BP Koirala Institute of Health Sciences, Dharan, Nepal.
Correspondence to: Dr. Rupa Rajbhandari Singh, Department of
Pediatrics and Adolescent Medicine, B.P. Koirala Institute of Health
Sciences, Dharan, Nepal.
Received: February 07, 2012;
Initial review: February 27, 2012;
Accepted: September 21, 2012.
Published online: 2012, October 5.
PII: S097475591200127
CTRI/2010/091/001061
|
Objective: To study the role of Zinc in the treatment of neonatal
sepsis.
Design: Double blind, randomized, placebo
controlled trial.
Setting: Tertiary Care Hospital.
Participants: 614 neonates with probable neonatal
sepsis.
Intervention: The drug group (n=307)
received 1mg/kg/day of elemental zinc, and placebo group (n=307)
received the placebo, in addition to antibiotic therapy and supportive
care, till the final outcome (discharge/death).
Outcome Measures: Decrease in mortality rates
(primary outcome), duration of hospital stay and need of higher lines of
antibiotic therapy (secondary outcomes) were tested.
Results: Baseline characteristics of the two
groups were similar. No statistically significant differences between
drug and placebo group were found in mortality rate (9.77% vs
7.81 %; P=0.393), mean duration of hospital stay (142.85±69.41 hrs,
vs. 147.99±73.13 hrs; P=0.841), and requirement of higher
lines of antibiotic therapy (13.35% vs 12.05%, P=0.628)
after supplementation.
Conclusions: This study does not report decrease
in mortality rates, duration of hospital stay and requirement of higher
lines of antibiotic therapy following zinc supplementation in neonatal
sepsis.
Key words: Mortality, Newborn, Outcome, Sepsis,
Zinc.
|
|
Despite advances in neonatal care, the mortality
and morbidity from neonatal sepsis still remains high. The reported
incidence of neonatal sepsis varies from 7.1 to 38 per 1000 live births
in Asia [1,2]. In Nepal, out of the total infant mortality rate of
46/1000 live births, more than two-third, i.e. 33/1000 live births is
contributed by neonatal mortality [3]. A similar situation exists in
India and other developing countries in South-East Asia [4].
Multiple factors contribute to the increased
susceptibility of neonates to infection. These include developmental,
quantitative and qualitative neutrophil defects, decreased bone marrow
neutrophil pool, and quantitative and qualitative deficiencies in
immunoglobulins [5]. Zinc is known to play a central role in the immune
system. It is crucial for normal development and function of cells
mediating innate immunity, neutrophils, macrophages and natural killer
cells. Phagocytosis, intracellular killing, cytokine production, and T
and B cell function are all affected by zinc deficiency [6,7]. Decreased
rates of infection have been observed following zinc supplementation in
several population-based studies of different diseases, notably diarrhea,
pneumonia and malaria [8].
Earlier studies of zinc supplementation in neonates
have shown significantly reduced mortality in small for gestational age
(SGA) infants [9], increased growth among low birth weight (LBW) [10,11]
and very low birth weight (VLBW) infants [12]. However, there have been
no published studies of zinc supplementation in neonates with sepsis.
This study was done to evaluate whether therapy with zinc in neonates
with sepsis would decrease mortality, lead to earlier discharge from
hospital, and decrease the requirement of higher lines of antibiotic
therapy.
Methods
This study was conducted in the Pediatric wards of BP
Koirala Institute of Health Sciences (BPKIHS), a level III tertiary care
hospital in the Eastern region of Nepal, between May 2010 and January
2011. A sample size of 614 was calculated to be sufficient to detect 50%
difference between study and control groups with 80% power and alpha of
0.05.
Ethical clearance was obtained from the Institutional
Ethical Review Board, BPKIHS. The procedures followed were in accordance
with the ethical standards of the responsible committee on human
experimentation and with the Helsinki Declaration of 1964, as revised in
2008. Written informed consent was taken from the parents of all
neonates enrolled in the study.
Intramural and extramural neonates of >32 weeks
presenting to BPKIHS with diagnosis of ‘Probable Neonatal Sepsis’ ’
during the study period, whose parents consented to be a part of the
study were included. Probable neonatal sepsis was defined as per the
guidelines [1] formulated by the National Neonatology Forum of India
based on the duly approved tenth revision of the International
Classification of Diseases (ICD) [13] by the WHO. In an infant having a
clinical picture suggestive of sepsis, the presence of any one of the
following criteria was considered enough to suspect the diagnosis of
bacterial infection [1,13]:
(a) Existence of any of the predisposing
factors like premature rupture of membranes (PROM), foul smelling
liquor, amnionitis/funisitis, gastric polymorphs >5/HPF.
(b) Positive ‘sepsis screen’ i.e. presence
of at least two of the following five parameters, namely, total
leucocyte count <5000/mm 3,
low absolute neutrophil count (as per standard charts), bands to
total neutrophil ratio (IT ratio) of >0.2, C-reactive protein>1mg/dL,
micro ESR >15mm in first hour on any day of life/age in days+3.
(c) Radiological evidence of pneumonia
In case of parental refusal to consent, neonates <32
weeks, neonates with severe birth asphyxia (5 min Apgar score <5),
congenital malformations and necrotizing enterocolitis, the neonates
were excluded from the study. The primary outcome measure was
mortality and the secondary outcomes were duration of hospital stay, and
requirement of higher lines of antibiotic therapy.
Randomization and blinding: The neonates who
enrolled into the study were randomized in two groups, the drug and
placebo group. The sequence used to enroll the neonates in either group
was generated by using restricted randomization by using the permuted
block design of 1:1 to ensure an equal sample size in either group. The
person who generated the sequence of drugs was not involved in
monitoring the study. Patients were allocated a specific numbered strip
of either zinc or placebo tablets, without revealing its identity. The
tablets were identical in appearance, consistency and taste. The
sequence of code numbers were kept in a sealed envelope which was opened
by a nursing officer, not a part of the study, who identified the groups
after the completion of the study. Stratification of the study
population was done on the basis of onset of neonatal sepsis. All study
participants and personnel including care providers, evaluators and
monitors were blinded to treatment assignment for the entire duration of
the study to avoid any kind of bias. Neither the study participants nor
the person distributing the medication were able to identify the
drug/placebo during the course of the study. Patient blinding was
evaluated by asking questions to the parents to indicate which type of
treatment they believed their baby had received, but no one was able to
identify the drug/placebo. Similarly, study personnel were asked
questions as to which formulation they thought they were providing to
the participants, but none could identify the drug/placebo correctly.
Intervention: A detailed history reviewing the
antenatal, natal and postnatal factors was taken and tabulated.
Gestational age of the neonates was estimated by using the Modified
Ballard scoring system. Weight was measured to 5 g with an electronic
scale (SECA Corporation, Columbia, MD). Baseline investigations like
sepsis screen, appropriate cultures, chest radiographs and lumbar
punctures were done as needed. In addition to antibiotics (Cefotaxime
and Gentamicin, first line, as per hospital protocol) and standard
supportive care, the neonates in the drug group received zinc at
1mg/kg/day dissolved in expressed breastmilk (formulation: Zinc Sulphate
Dispersible tablets of 10 mg) either orally or via a nasogastric tube in
neonates kept NPO, while the neonates in the placebo group received a
safe placebo. In both groups, the formulation was given till the final
outcome (discharge/death). During the course of therapy, the neonates
were evaluated daily and in case the need to revert to higher lines of
antibiotic therapy (2 nd
line, Ceftazidime and Amikacin; 3rd
line, Vancomycin and Meropenem; as per hospital protocol), the decision
to do so was taken by the treating pediatricians.
Statistical analysis: The data obtained
was entered into Microsoft Excel. All analysis was carried out using the
statistical software SPSS (Version 16; SPSS Inc., Chicago, IL). Pearson
Chi-square test and Fisher exact test were used to test for statistical
significance between the parameters and clinical criteria. Odds ratio
was used for comparison of case and control. Two sided significance
tests were used throughout and P value <0.05 was considered to be
statistically significant.
Results
A total of 1540 neonates with probable neonatal
sepsis were admitted during the study period, of which 840 were
excluded. Of the remaining 700 neonates, 86 were lost to follow-up and a
total of 614 neonates with probable neonatal sepsis were analyzed in
this trial. Of these 614 neonates, half received zinc and the other half
received a placebo along with standard antibiotic therapy and supportive
care (Fig 1). No side-effects were noted in either group.
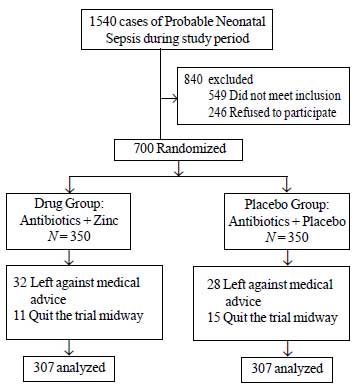 |
|
Fig.1 Study flow-chart.
|
The baseline characteristics of both the groups were
similar (Table I). The neonates enrolled in the two groups
had similar risk factors for neonatal sepsis. Culture/sensitivity and
sepsis screen outcomes were also similar in the two groups.
TABLE I Baseline Characteristics In The Two Groups
Characteristics
|
Drug Group
(n=307) |
Placebo Group
(n=307) |
|
Male |
193 |
200 |
|
Gestational age (wk)* |
38.05 ± 2.11 |
38.11 ± 2.11 |
|
Apgar@1 min* |
6.56 ± 1.46 |
6.76 ± 1.32 |
|
Apgar@5 min* |
7.80 ± 0.98 |
7.93 ± 1.01 |
|
Apgar@10 min* |
8.73 ± 0.85 |
8.83 ± 0.81 |
|
Early onset sepsis |
256 |
244 |
|
Late onset sepsis |
51 |
63 |
|
Intramural patients |
166 |
141 |
|
Cesarean delivery |
65 |
68 |
|
Vaginal delivery |
232 |
230 |
|
Vehicle delivery |
10 |
9 |
|
Birth weight (g)* |
2461.7 ± 640.16 |
2652.9 ± 652.72 |
|
* Values in mean (SD) |
When the final outcome in the two groups was compared
(Table II), we found that 30 neonates in the drug group
and 24 in the placebo group expired (P=0.393). Further, 41
neonates in the drug group as compared to 37 in the placebo group
required higher lines of antibiotic therapy (P=0.628). The mean
duration of hospital stay, 142.85 ± 69.41 hours in the drug group as
compared to 147.99 ± 73.13 hours in the placebo group was also not
statistically significant (P = 0.841).
TABLE II Comparison between Drug and Placebo Groups Based on Outcome Measures
|
Measure |
Drug Group |
Placebo Group
|
|
(n=307)
No. (%) |
(n=307)
No. (%) |
|
Mortality |
30 (9.77) |
24 (7.81) |
|
Use of 2/3 line antibiotics |
41 (13.35) |
37 (12.05) |
|
Hospital stay (hrs)* |
142.85 (69.41) |
147.99 (73.13) |
|
* Values in mean (SD); P >0.05 for all three measures in
column 1. |
Discussion
We aimed to study the role of zinc in the treatment
of neonatal sepsis. After the analysis of 614 neonates with probable
neonatal sepsis, we did not find any significant difference in terms of
decrease in mortality rate, duration of hospital stay and requirement of
higher lines of antibiotic therapy between the zinc supplemented and
placebo groups.
We would like to emphasize on the fact that till
date, there are no published studies to evaluate the role of zinc in
neonatal sepsis. The strengths of this study included its robust
randomization, allocation concealment, double-blind design, large sample
size with adequate power and homogeneity between the two groups, all of
which add to the internal validity of the study. The larger
interpretation of this study would need to consider some potential
limitations which include this being a single center study and inability
to estimate serum zinc levels to rule out any underlying zinc deficiency
prior to the onset of therapy. Also, there is paucity of literature
regarding the exact role of zinc on the neonatal immune system, a clear
understanding of which would aid in designing such studies.
As this study is the first of its kind reported in
literature, we were unable to compare the results with those obtained in
other similar studies. The lack of significant differences between the
zinc supplemented and placebo groups in this study could be explained by
the fact that the duration of therapy in the neonates in both arms of
the trial was not standardized (the formulation was given till the
subject was discharged/expired, and not for a standardized unit of time
to both arms). The duration of treatment (mean, 145 hours) may not have
been sufficient enough for zinc to significantly augment the immune
system. Lack of a significant role of zinc in augmenting the neonatal
immune system in particular, and a possible absence of zinc deficiency
in the neonates enrolled in our trial could also be other reasons for
these findings.
Based on the findings of the present study, it can be
concluded that there was no difference in terms of decrease in mortality
rate, duration of hospital stay and requirement of higher lines of
antibiotic therapy between the zinc supplemented and placebo groups of
neonates. Further studies conducted after a thorough understanding of
the role of zinc on the neonatal immune system, incorporating paired
sample observations of serum zinc levels (before and after
supplementation) and subsequently meta-analyses are required to further
clarify the role of zinc in the treatment of neonatal sepsis.
Acknowledgments: Mr. D. D. Baral and Dr. Adithya
P. for their help with statistical analysis.
Contributors: KM, NKB and RRS conceived and
designed the study and revised the manuscript for important intellectual
content. KM & RRS will act as guarantors of the study. SM conducted the
laboratory tests and helped interpret them. KM, NKB and MKS analyzed the
data and helped in the preparation of the manuscript. The final
manuscript was approved by all authors.
Funding: Deurali Janta Pharmaceuticals Limited,
Nepal provided the Zinc and the Placebo used in the study.
Competing interests: None stated.
|
What is Already Known?
• Zinc supplementation reduces rates of
diarrhea and pneumonia in young infants.
What this Study Adds?
• This study did not find any benefit of oral zinc
supplementation for the treatment of probable neonatal sepsis.
|
References
1. Singh M, Paul VK, Bhakoo ON. Neonatal Nomenclature
and Data Collection. New Delhi: National Neonatology Forum;
1989.p.63-74.
2. Vergnano S, Sharland M, Kazembe P, Mwansambo C,
Health PT. Neonatal sepsis : an international perspective. Arch Dis
Child Fetal Neonatal. 2005;90:F220-4.
3. Nepal Demographic and Health Survey 2011 –
Preliminary report. MOHP, Govt. of Nepal. Available from: URL:
http://www.mohp.gov.np/english/publication
/NDHS%20%202011%20Preliminary%20Report.pdf. Accessed July 15, 2012.
4. State of World Children. UNICEF report. Available
from: URL: http://www.unicef.org. Accessed July 15, 2012.
5. Stoll BJ, Kleigman RM. The fetus and the neonatal
infant. In: Behrman RE, Kliegman RM, Jenson HB, Nelson Textbook
of Pediatrics. 17ed, Philadelphia: WB Saunders Co; 2004;552;623-39.
6. Shankar AH, Prasad AS. Zinc and immune function:
the biological basis of altered resistance to infection. Am J Clin Nut.
1998;68,447S-463S.
7. Prasad AS. Zinc in human health: effect of zinc on
immune cells. Mol Med. 2008; 14:353-7.
8. IZiNCG. Systematic reviews of zinc intervention
strategies. International Zinc Nutrition Consultative Group Technical
Document #2. Brown KH, Hess SY, editors. Food Nutr
Bull. 2009;30:S1-S184.
9. Sazawal S, Black RE, Menon VP, Dinghra
P, Caulfield LE, Dhingra U, et al. Zinc supplementation in
infants born small for gestational age reduces mortality: a prospective,
randomized, controlled trial. Pediatrics. 2001;108:1280-6.
10. Castillo-Duran C, Rodriguez A, Venegas G, Alvarez
P, Icaza G. Zinc supplementation and growth of infants born small for
gestational age. J Pediatr. 1995;127:206-11.
11. Islam MN, Chowdhury M, Siddika M,Qurishi SB, Bhuiyan
MK, Hoque MM, et al. Effect of oral zinc supplementation on the
growth of preterm infants. Indian Pediatr. 2010;47:845-9.
12. Friel JK, Andrews WL, Matthew JD, Long DR, Cornel
AM, Cox M, et al. Zinc supplementation in very-low-birth-weight
infants. J Pediatr Gastroenterol Nutr. 1993;17:97-104.
13. International Classification of Diseases (ICD), 10th revision,
1989. Geneva: World Health Organization; 1989.
|
|
|
 |
|

