|
|
|
Indian Pediatr 2013;50: 377-381
|
 |
Lactobacillus rhamnosus GG
Supplementation for Preventing Respiratory Infections in
Children: A Meta-analysis of Randomized, Placebo-controlled
Trials
|
|
Shan Liu, PengWei Hu, Xiaoxin Du, Tao Zhou
and Xiaofang Pei
From the Department of Public Health
Microbiology, Department of Public Health Laboratory Technology,
West China School of Public Health, Sichuan University, China.
Correspondence to: Xiaofang Pei, Professor of
Public Health Microbiology, West China School of Public Health,
Sichuan University, 16#, Section 3, Renmin Nan Lu, Chengdu,
Sichuan 610041, China.
Email: [email protected]
|
Objective:
To systematically
review the effectiveness of administering Lactobacillus rhamnosus
GG (LGG) for preventing respiratory infections in children.
Design: Systematic Review and Meta-analysis.
Data sources: Electronic databases and trial
registries.
Results: Four RCTs involving 1805 participants
met the inclusion criteria. Compared with placebo, LGG administration
was associated with a reduced incidence of acute otitis media (four
RCTs, n=1805, RR 0.76, 95% CI 0.64-0.91, fixed effects model, NNT 17,
95% CI 11-46), a reduced risk of upper respiratory infections (one RCT,
n=281, RR 0.62, 95% CI 0.50-0.78, NNT 4, 95% CI 3-8) and antibiotic
treatments (four RCTs, n=1805, RR 0.80, 95% CI 0.71-0.91, fixed effects
model). There was no significant difference between the LGG and the
control groups in the risk of overall respiratory infections and the
incidence of lower respiratory infections. However, subgroup analysis of
two studies on children older than 1 year showed significant reduction
in the risk of overall respiratory infections (two RCTs, n=794, RR 0.73,
95% CI 0.57-0.92, random effects model, NNT 8, 95% CI 5-14). Adverse
effects were similar in both groups. No serious adverse events were
reported.
Conclusion: The administration of
Lactobacillus rhamnosus GG compared with placebo has the potential
to reduce the incidence of acute otitis media, the upper respiratory
infections and antibiotic use in children.
Key words: Children, LGG, Prevention. Probiotics,
Respiratory infections.
|
|
Respiratory (tract)
infections are common among children and
contribute substantially to pediatric morbidity
and mortality worldwide. The inappropriate use
of antibiotics for the treatment of these
infections can cause side effects in children,
including rash, diarrhea, and increased
bacterial drug resistance rates [1]. Prevention
of respiratory tract infections is an important
publichealth challenge. A safe, relatively
inexpensive, and effective intervention to
prevent respiratory tract infections and its
adverse effects to health would have significant
public-health implications.
In this era of increasing
antimicrobial resistance, use of probiotics in
infection prevention has brought a new
perspective. Probiotics have been defined as
"live microorganisms which when administered in
adequate amounts confer a health benefit on the
host" [2]. One of the most studied probiotics is
Lactobacillus rhamnosus GG (LGG), which
influences the immune response both by
stimulating antibody production and by improving
the phagocytic activity of the blood leucocytes
[3]. In children, there is now convincing data
to support the use of LGG for the treatment of
abdominal pain-related functional
gastrointestinal disorders and the prevention of
diarrhea [4]. Some studies show that probiotic
strains can prevent respiratory infections [5].
However, evidence for the role of LGG in
preventing respiratory tract infections in
children is not clear.
We conducted a systematic
analysis of data from all the currently
available trials to evaluate the evidence for
the efficacy of LGG in preventing respiratory
infections in children.
Methods
Inclusion and exclusion
criteria: All randomized controlled trials
to investigate the effect of LGG supplementation
in the prevention of respiratory infections (as
defined by the investigators) in children were
included. Participants were the children aged 0
month to 18 years who were from community. The
intervention was LGG, or LGG together with other
probiotics at any form or dose compared with
placebo or with no additional intervention. The
primary outcome measure was the incidence of
respiratory infections using the original
investigatorís definition, including the overall
respiratory infections, the upper and lower
respiratory infections and acute otitis media.
The secondary outcome measures were the
incidence of antibiotic treatments and the
adverse effects. We excluded studies of adults
and studies with participants who are
susceptible to infections. Only studies with
>80% follow-up were included.
Search methods: We tried
to identify all relevant trials irrespective of
language or publication status (published,
unpublished, in press and in progress). We
systematically searched the major electronic
databases (MEDLINE, EMBASE, ISIís Web of
Science, the Cochrane Library and Chinese
Publications) from their inception to September
2012 using the following terms with a
topic-specific strategy: [respiratory infections
OR respiratory tract infections] AND [probiotic(s)
OR lactobacillus OR LGG OR L rhamnosus] AND
[child(ren) OR infant(s) OR baby OR adolescent
OR teenager]. Besides, two trial registries
(ClinicalTrials.gov and EU Clinical Trials
Register) were searched through their websites.
Selection of studies: Two
authors (SL and PH) checked independently the
titles and abstracts recognized via the search
to identify the potentially eligible relevant
publications and obtained the full articles.
Then the articles were estimated by the same two
authors utilizing an eligibility form based on
the inclusion criteria. If there was an
uncertainty whether the study should be included
in the review, we attempted to contact the study
author for clarification. All differences in
opinion were resolved by further discussion or
by discussion with a third author (XP). We
excluded studies that did not meet the inclusion
criteria and presented the reasons for their
exclusion.
Data extraction and
management: Data on author, year of
publication, study methods, participants,
interventions and outcome measures were
extracted independently by two authors (SL and
PH) according to a standardized data extraction
form. Any disagreement among authors was
resolved by discussion and review of the
original publication. Data were then imported
into the Cochrane Review Manager 5. For
dichotomous outcomes, we extracted the total
number of participants and the number of
participants with the event for each group. For
continuous outcomes, we extracted the total
number of participants, geometric means and
standard deviations. We compared the extracted
data to identify errors.
Assessment of risk of bias in
included studies: SL and PH independently
assessed the risk of bias of the included trials
using the current version of the Cochrane
Handbook [6]. Any discrepancies were resolved by
discussion. Randomization (sequence generation),
blinding of participants and assessors,
allocation concealment and incomplete data
outcome were examined.
Data synthesis: We
analyzed the data using Review Manager 5. For
dichotomous data, the outcomes were analyzed as
a comparison of proportions using risk ratio
(RR) as a measure of effect. The mean difference
was selected to represent the difference for
continuous data. All results were presented with
95% confidence intervals (CI). Heterogeneity of
effect sizes among the different trials was
assessed by inspection of the forest plot using
the chi-squared statistic and I 2
statistic. We combined the
data using a fixed effect model. Where there was
the heterogeneity (I2>50%),
and it was still appropriate to combine trials,
we used the random effects model. To investigate
heterogeneity, we analyzed subgroups according
to the different ages of participants in some
outcomes of the review. The effectiveness was
also expressed as the "numbers needed to treat"
(NNT) with a 95% CI to prevent a case of
respiratory infections, which was calculated by
STATSDIRECT statistical software (version 2.7.8,
2010-11-8; StatsDirect Ltd., Altrincham, UK).
Results
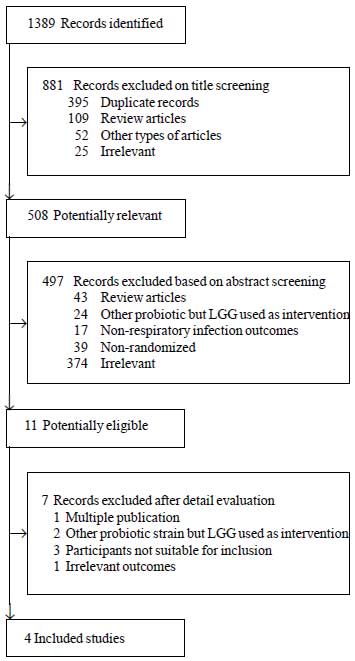
The flowchart of article
selection is shown in Fig. 1. A total of
1389 studies were identified from the primary
electronic databases. After independent
assessment of the titles and abstracts, 1378
were excluded as a result of duplicates (n=395),
review articles (n=152), irrelevant (n=699),
etc. Subsequently, authors independently
reviewed the full texts of the remaining 11
articles and indentified that four studies met
the inclusion criteria [7-10]. Excluded studies
[11-17] are described in
Web Table I.
 |
|
Fig. 1
Flowchart of article selection.
|
The included four randomized
placebo-controlled trials consisted of 2135
participants, with 1805 evaluated. Risk of bias
assessment and the characteristics of the
included trials are presented in
Web Table
II. All studies were based in European
countries (Finland and Croatia) published during
2001 to 2010. The form of administration of
probiotics was milk supplemented with probiotics
or probiotics in capsules. In all studies, the
probiotics intervention group contained LGG and
was compared with placebo control group. In two
studies, LGG was the only intervention [7, 8].
In the others, LGG was administered together
with other probiotics (L. rhamnosus LC
705, Bifidobacterium breve 99,
Propionibacterium freudenreichii ssp shermanii
JS or Bifidobacterium Bb-12).The dose
of LGG and duration of intervention varied (Web
Table II). One trial assessed the
incidence of infections before the age of 7
months and the recurrent infections during the
first year of life in its intervention period
[10], while the remaining trials assessed the
incidence of infections or other outcomes for
the whole intervention period.
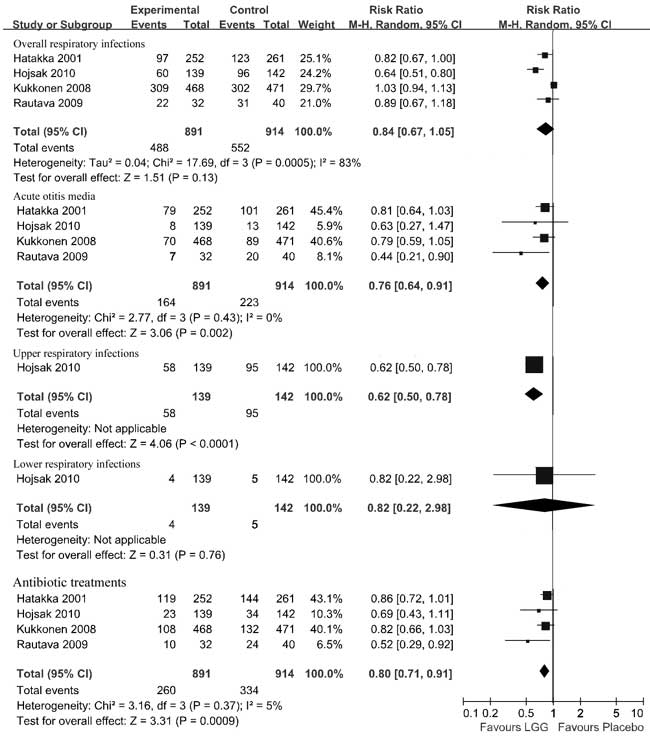
Primary outcomes:
Compared with the placebo group, the pooled data
in the LGG group had a significantly reduced
risk of acute otitis media (four RCTs, n=1805,
RR 0.76, 95% CI 0.64-0.91, fixed effects model,
NNT 17, 95% CI 11-46) and a reduced risk of
upper respiratory infections (one RCT, n=281,
RR 0.62, 95% CI 0.50-0.78, NNT 4, 95% CI 3-8).
Compared with the placebo group, children in the
LGG group had no significant reduction in risk
of the overall respiratory infections (four
RCTs, n=1805, RR 0.84, 95% CI 0.67-1.05,
random effects model) as well as no significant
reduction in the risk of lower respiratory
infections (one RCT, n=281, RR 0.82, 95%
CI 0.22-2.98). Significant heterogeneity was
found for the overall respiratory infections ( c2=17.69,
P=0.0005, I2=83%).
No significant heterogeneity was found for acute
otitis media (c2=2.77,
P=0.43, I2=0%)
(Fig. 2). For a subgroup of
children older than 1 year, the overall
respiratory infections was reduced in those in
the LGG group compared with those in the placebo
group (two RCTs, n=794, RR 0.73, 95% CI
0.57-0.92, random effects model, NNT 8, 95% CI
5-14) with heterogeneity (c2=2.60,
P=0.11, I2=62%).
For children younger than 2 months, there were
no differences in the overall respiratory
infections between the groups that received LGG
or placebo (two RCTs, n=1011, RR 1.02,
95% CI 0.93-1.11, fixed effects model) and no
heterogeneity (c2=0.94,
P=0.33, I2=0%).
 |
|
Fig.2 Effect
of Lactobacillus GG on respiratory
infections.
|
Secondary outcomes: The
pooled data showed a statistical significance
for reducing antibiotic treatments in the LGG
group compared with the placebo group (four
RCTs, n=1805, RR 0.80, 95% CI 0.71-0.91,
fixed effects model). No significant
heterogeneity was detected ( c2=3.16,
P=0.37, I2=5%)
(Fig.2). In two trials [7,8], no
adverse effects were reported, and both
products, LGG and placebo, were well tolerated.
Three infants receiving placebo experienced
vomiting, flatulence and increased fussing [10].
In the trial on newborn infants, the symptoms
included abdominal discomfort, vomiting, crying,
difficulty in swallowing the product and
noncompliance with no difference between the LGG
group and the placebo group [9].
Discussion
This meta-analysis of data
from RCTs showed that the use of probiotic
microorganism, LGG, compared with placebo among
children was associated with a reduction in the
incidence of acute otitis media, upper
respiratory infections and antibiotic
treatments. Subgroup analysis of two trials
conducted in children older than 1 year showed
significant reduction in the risk of overall
respiratory infections. With respect to safety,
no serious adverse effects were detected in the
included studies. Adverse effects were similar
in both groups.
Respiratory infections are
generally considered to include infections of
the lower and upper respiratory tract. However,
the definitions of outcome measures among
studies varied. In the trial by Hatakka, et
al. [7], acute otitis media and sinusitis
were reported as upper respiratory infections
and acute bronchitis and pneumonia as lower
respiratory infections [7]. One trial defined
rhinitis, pharyngitis, sinusitis, otitis, common
cold as upper respiratory tract infections and
pneumonia, bronchitis, bronchiolitis as lower
respiratory infections [8]. The other two trials
did not provide a definition at all [9,10]. Our
study indicated LGG may have a beneficial effect
for preventing the upper respiratory infections.
However, it did not have an effect on lower
respiratory infections, perhaps due to the small
number of infections affecting the lower
respiratory tract (4 in LGG group and 5 in
placebo group) [18,19]
To our knowledge, this is the
only meta-analysis that examines the effects of
LGG supplementation for the prevention of
respiratory infections in children. In many
countries, children experience three to six
respiratory infections a year and 40% of them
could even suffer from at least one episode of
acute otitis media which is one of the most
common bacterial infections and the main reason
for antibiotic treatment in childhood [20-22].
Thus, a 5-10≠% reduction in the incidence of
acute otitis media and antibiotic use, which our
results indicate is possible, could have
important clinical, public health, and economic
consequences.
Current data shows that
consumption of LGG appears to be an effective
strategy for reducing the risk of acute otitis
media and upper respiratory infections in
basically healthy children. In otitis-prone
children, who experience nasopharyngeal
colonisation of otitis pathogens, Hatakka, et
al. [14] indicated that LGG treatment did
not reduce the occurrence of acute otitis media.
This analysis did not have the ability to
evaluate the effect of LGG in preventing
respiratory infections among children who have
nasopharyngeal colonization with pathogens.
Contributors: LS searched
literature, extracted data, conducted analysis
and drafted manuscript. HPW searched literature,
extracted data and conducted analysis. DXX and
ZT checked the manuscript. PXF planned the study
and contributed to manuscript writing.
Funding: None;
Competing interest: None stated.
References
1. Williams BG, Gouws E,
Boschi-Pinto C, Bryce J, Dye C. Estimates of
world-wide distribution of child deaths from
acute respiratory infections. Lancet Infect Dis.
2002; 2:25-32.
2. Goldin BR, Gorbach SL.
Clinical indications for probiotics: An
overview. Clin Infect Dis. 2008; 46: S96-S100.
3. Saxelin M. Lactobacillus
GG - a human probiotic strain with thorough
clinical documentation. Food Rev Int.
1997;13:293-313.
4. Horvath A, Dziechciarz P,
Szajewska H. Meta-analysis: Lactobacillus
rhamnosus GG for abdominal pain-related
functional gastrointestinal disorders in
childhood. Aliment Pharm Ther. 2011;33:1302-10.
5. Sanz JMC, Mateos JA,
Conejo AM. Effect of Lactobacillus casei
on the incidence of infectious conditions in
children. Nutr Hosp. 2006;21:547-51.
6. Higgins JPT, Green S,
editors. Cochrane Handbook for Systematic
Reviews of Interventions Version 5.1.0 [updated
March 2011]. The Cochrane Collaboration, 2011.
Available from www.cochrane-handbook.org.
7. Hatakka K, Savilahti E,
Ponka A, Meurman JH, Poussa T, Nase L, et al.
Effect of long term consumption of probiotic
milk on infections in children attending day
care centres: Double blind, randomised trial.
Brit Med J. 2001;322:1327-9.
8. Hojsak I, Snovak N,
Abdovic S, Szajewska H, Misak Z, Kolacek S.
Lactobacillus GG in the prevention of
gastrointestinal and respiratory tract
infections in children who attend day care
centers: A randomized, double-blind,
placebo-controlled trial. Clin Nutr.
2010a;29:312-6.
9. Kukkonen K, Savilahti E,
Haahtela T, Juntunen-Backman K, Korpela R,
Poussa T, et al. Long-term safety and
impact on infection rates of postnatal probiotic
and prebiotic (synbiotic) treatment: Randomized,
double-blind, placebo-controlled trial.
Pediatrics. 2008;122: 8-12.
10. Rautava S, Salminen S,
Isolauri E. Specific probiotics in reducing the
risk of acute infections in infancy - a
randomised, double-blind, placebo-controlled
study. Brit J Nutr. 2009;101: 1722-6.
11. Hojsak I, Abdovic S,
Szajewska H, Milosevic M, Krznaric Z, Kolacek S.
Lactobacillus GG in the prevention of nosocomial
gastrointestinal and respiratory tract
infections. Pediatrics. 2010b;125:E1171-7.
12. Smerud HK, Kleiveland CR,
Mosland AR, Grave G, Birkeland S-E. Effect of a
probiotic milk product on gastrointestinal and
respiratory infections in children attending
day-care. Microbial Ecology to Health and
Disease. 2008;20:80-5.
13. Kekkonen RA, Vasankari
TJ, Vuorimaa T, Haahtela T, Julkunen I, Korpela
R. The effect of probiotics on respiratory
infections and gastrointestinal symptoms during
training in marathon runners. Int J Sport Nutr
Exe. 2007;17: 352-63.
14. Hatakka K, Blomgren K,
Pohjavuori S, Kaijalainen T, Poussa T, Leinonen
M, et al. Treatment of acute otitis media
with probiotics in otitis-prone children - a
double-blind, placebo-controlled randomised
study. Clin Nutr. 2007;26:314-21.
15. Weizman Z, Asli G,
Alsheikh A. Effect of a probiotic infant formula
on infections in child care centers: Comparison
of two probiotic agents. Pediatrics.
2005;115:5-9.
16. Schrezenmeir J, Heller K,
McCue M, Llamas C, Lam W, Burow H, et al.
Benefits of oral supplementation with and
without synbiotics in young children with acute
bacterial infections. Clin Pediatr.
2004;43:239-49.
17. Coulthard MG, Mellis CM.
Does probiotic milk prevent infections in
children attending daycare centres? Med J
Australia. 2004;181:556-7.
18. Spindler-Vesel A,
Bengmark S, Vovk I, Cerovic O, Kompan L.
Synbiotics, prebiotics, glutamine, or peptide in
early enteral nutrition: A randomized study in
trauma patients. Jpen-Parenter Enter.
2007;31:119-26.
19. Rayes N, Hansen S,
Seehofer D, Muller AR, Serke S, Bengmark S,
et al. Early enteral supply of fiber and
lactobacilli versus conventional nutrition: A
controlled trial in patients with major
abdominal surgery. Nutrition. 2002;18: 609-15.
20. Laurent C, Dugue AE,
Brouard J, Nimal D, Dina J, Parienti J-J, et
al. Viral epidemiology and severity of
respiratory infections in infants in 2009 a
prospective study. Pediatr Infect Dis J.
2012;31: 827-31.
21. Vesa S, Kleemola M,
Blomqvist S, Takala A, Kilpi T, Hovi T.
Epidemiology of documented viral respiratory
infections and acute otitis media in a cohort of
children followed from two to twenty-four months
of age. Pediatr Infect Dis J. 2001;20:574-81.
22. Alho OP, Koivu M, Sorri
M, Rantakallio P. The occurrence of acute otitis
media in infants. A life-table analysis. Int J
Pediatr Otorhinol. 991;21:7-14.
|
|
|
 |
|

