|
Desferoxamine (DFO), the first
effectively
utilized iron chelator produced a
dramatic effect on survival of patients
with thalassemia. However, DFO has poor oral bioavailability and a short
half life, necessitating 12 hours of subcutaneous infusion, rendering
therapy extremely cumbersome [1]. Deferiprone (DFP), an orally effective
iron chelator, reduces total body iron load and is also effective in
removing cardiac iron [1-3]. However, it has a short life and needs to
be taken thrice daily, besides having troublesome side effects of
arthralgia and neutropenia. Combination of DFO and DFP has proved to be
more effective in reducing cardiac iron overload than DFO alone in
clinical trials [4]. Deferasirox (DFX), a recently approved,
efficacious, safe, oral iron chelator has the advantage of a longer
half life and hence requires once daily administration, leading to
better compliance [5,6].
Non-invasive quantification of myocardial iron can be
done using cardiovascular magnetic resonance (CMR) by measuring
myocardial T2*. MRI T2* is a measure of magnetic relaxation which is
easier to measure than T2 and the extent of cardiac iron on MRI T2*
provides useful insight into the severity of myocardial siderosis [7].
T2* gradient echo measures decay in signal intensity as the echo time of
images progressively increases. This rate of decay is enhanced in
presence of iron deposition.
Serum ferritin concentration is a convenient,
non-expensive and the most widely used measure of assessing total body
iron but is a poor predictor of cardiac iron status [8,9]. We
prospectively assessed serum ferritin levels and myocardial T2* in an
enrolled group of multi-transfused thalassemia patients to evaluate the
efficiency of DFX as an iron chelator.
Methods
This prospective single arm study was conducted
between October 2008 and October 2010, on 30 multi-transfused
thalassemic patients to monitor the effect of DFX on myocardial and
total body iron load. To quantify change in cardiac iron load,
myocardial T2* was measured at baseline and then after 12-18 months of
DFX therapy, on a 1.5 Tesla Siemens Sonata machine. Though all chelating
agents have a short half-life of less than a day, a washout period of a
week was given before starting DFX to avoid any confounding. Patients
were scanned using a single 8 mm thick, short axis mid ventricular
slice, acquired at 8 different echo times. End systolic and diastolic
ventricular volume and ejection fraction (EF) were measured using a
standard reproducible CMR sequence as per published norms [10,11]. For
T2* measurement we used the software CMRtools created by Imperial
College and utilized the Argus software on the Siemens workstation for
EF evaluation. Cardiac T2* value of <20 ms is indicative of iron
overload as below this value there is progressive decline in the LV
function. Values of <10ms are suggestive of severe cardiac siderosis
[7,10,11]. EF of <56% was considered to be significant cardiac
dysfunction and such patients were not included in this study as
continuous DFO or combined DFO+DFP form the standard care for them [12].
All 30 patients studied were asymptomatic from cardiac prospective. The
radiologists performing MRI T2* scan were blinded to the details of
therapy of the patients.
Serum ferritin (SF) level was estimated by ELISA from
pretransfused blood sample when the first MRI was done i.e. pre DFX
therapy and subsequently every 3 months. Urine examination was done
every month for albuminuria, serum creatinine and ALT levels were
estimated monthly for the first 3-6 months, and subsequently every 3
months. All adverse events were documented. Patients were started on
single dose DFX at 20-25 mg/kg/day given on an empty stomach in the
morning and further dose escalation done to a maximum of 35 mg/kg/day.
Dose reduction was done if any side effect was noted or if serum
ferritin fell below 500 ng/mL. This study was conducted in accordance
with Good Clinical Practice guidelines and was approved by the Hospital
Ethical Committee. Informed consent was obtained from parents, guardian
or patients themselves. The primary end point of the trial was to
evaluate the difference in serum ferritin and cardiac MRI T2* after
12-18 months of DFX therapy as compared to baseline values. Data are
presented as mean ± SD and variables analyzed by paired and unpaired
t- test for statistical significance. P <0.05 was considered
statistically significant.
Results
There were 30 patients (22 males, 73.3%) receiving
regular transfusion with a mean age of 15.7 ±6.8 years (range 6.5 to 29
years) and average weight of 34.2±12.5kg. The mean blood transfusion
requirement was 219.4 mL/kg/year with a range of 180-260 mL/kg/year and
median transfusion duration was 180 months (range 96-310 months). 6
patients (20%) had baseline T2* <10ms, 9 (30%) had T2* between 10 to 20
ms while 15 (50%) patients had T2* >20ms. All the patients were started
on DFX 20mg/kg/day initially but due to non appreciable decline in serum
ferritin, required upgradation to 30 mg/kg/day dose over next 6 month.
Only 4 of our patients required 35 mg/kg/day dose for control of
ferritin level. Table I shows the serum ferritin, cardiac
iron load (as T2*) and cardiac functions before and after deferasirox
therapy. Table II shows the mean and percentage change of
T2* in various risk groups and their corresponding serum ferritin change
(Fig.1).
TABLE I Change in Serum Ferritin, Cardiac Iron (T2*) and Cardiac Function Pre and Post Deferasirox Therapy
| Parameter |
Pre therapy
Mean ± SD (range) |
Post therapy
Mean ± SD (range) |
% change
|
P value |
| Serum ferritin
(ng/mL) |
3859.8 ±
1690.7
(1066 – 6725) |
2693.4 ±
1831.5
(660-8702) |
30.2
|
0.001 |
| Cardiac T2*
(ms)
|
23.8±15.2
(6.2- 69.2) |
24.2 ± 12.9
(7.6-48.5) |
1.6
|
0.870 |
| Ejection
fraction (%)
|
62.0 ±7.0
|
58.9 ± 4.8 |
4.9 |
0.061
|
| End
diastolic volume (mL) |
84.9 ±31.8 |
108.2 ±42.0 |
27.5 |
0.001
|
| End
systolic volume (mL) |
32.2 ±14.3
|
44.8 ±19.7
|
39.1 |
0.001 |
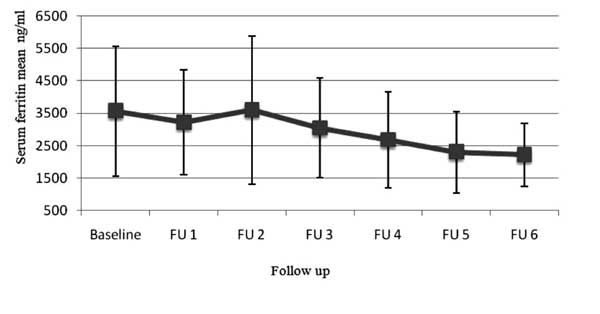 |
|
Fig. 1 Mean value of serum ferritin on
follow-up in patients treated with deferasirox.
|
TABLE II Change in Serum Ferritin and Cardiac T2* Before and After Treatment with Deferasirox (DFX)
Cardiac
T2* (ms) |
Pre treatment |
Post treatment |
Mean change |
P
|
Serum Ferritin
|
Mean and |
P
|
| |
Mean ± SD |
Mean ± SD |
and (%change) |
value
|
Pre
treatment |
Post
treatment
|
(% change) |
value
|
| |
|
|
|
|
Mean± SD
|
Mean±
SD
|
after
treatment |
|
|
<10ms (n=6) |
8.3±1.3 |
10.4±2.8 |
2.1 (24.8%) |
0.053
|
4568.8±1867.6 |
2402.0±354.5 |
1027.3 (22.5)
|
0.008
|
|
10-20ms (n=9) |
14.5±3.5 |
19.3±8.3 |
4.8 (33.4%) |
0.058
|
4271.1±1584.1 |
2505.8±1434.0 |
1765.3 (41.3) |
0.0001
|
|
>20ms (n=15) |
35.6±12.7 |
32.6±11.3 |
3.0 (8.4%) |
0.493
|
3329.4±1617.4 |
2466.7±1823.9 |
862.7 (25.9) |
0.002
|
|
Total (n=30) |
23.8±15.2 |
24.2±12.9 |
0.4 (1.6%) |
0.870 |
3859.8±1690.7 |
2693.4±183.4 |
1166.4 (30.2) |
0.001 |
Significant decrease in serum ferritin also occurred
in those with cardiac T2* <10 ms and between 10 to 20 ms (P<0.05).
In the subgroup of patients with cardiac T2* <10ms and between 10 to 20
ms, there was a greater improvement in cardiac iron overload with a 24.8
% and 33.4% increase in T2* value from the baseline, indicating greater
reduction of cardiac iron overload in this group as compared to mildly
iron overloaded patients who had 8.4% improvement in T2* (although both
these value were not statistically significant).
Discussion
The outcome of patients with cardiac siderosis cannot
be predicted on the basis of serum ferritin as ferritin is not a
suitable predictor of subclinical cardiac disease and cardiac
decompensation can occur with serum ferritin level <2500 ng/mL [8,9].
This may be due to the fact that iron chelators (including deferasirox)
remove iron from liver more rapidly than from the heart, and also
the possible genetic variations of various cardiac ion transport
channels [7]. Measurement of cardiac function by echocardiography
is not accurate in predicting cardiac dysfunction as in thalassemia,
cardiac function is supra normal and decline in systolic function is a
late sign of cardiac siderosis. Once cardiac dysfunction occurs,
there is high risk of death, unless chelation is dramatically
intensified [13]. Cardiac T2* is the best predictor of congestive heart
failure (CHF) and of arrhythmias in patients with cardiac siderosis.
With T2* <6 ms, approxi-mately 50% of patients develop CHF within 1
year. The cardiac T2* is also a good predictor of arrhythmia as well as
of CHF as approximately 90% of CHF patients have T2* <10 ms
whereas about 83% of patients with arrhythmia have cardiac T2*<20
ms [7,17,18].
In our study DFX not only decreased total body iron,
i.e. decrease in serum ferritin, but also effectively chelated
cardiac iron, particularly in children with T2* <10 ms. The current dose
of DFX approved by most authorities is 30mg/kg/day [14,15]. Although the
optimal dose for cardiac iron chelation has not been fully defined, FDA
and other health authorities recently approved doses up to 40mg/kg/day
in those patients whose cardiac iron overload is not controlled on
standard recommended doses [8,15]. However, even with good compliance,
some patients are known to respond poorly to DFX therapy due to decrease
in drug bioavailability at higher dose [12].
Although there was an improvement in cardiac T2*
after 12-18 month of therapy, this was statistically not significant.
This could be due to small sample size and the fact that we started DFX
at 20mg/kg/dose initially and increased gradually up to max of
35mg/kg/day depending on the need and tolerance; hence the patient’s
exposure to optimum dose was short. A clear demarcation between the good
responders (i.e. those with T2* <20 ms) and not so good responder
was oberved. None of our patient with low risk i.e. T2*>20 ms
progressed to moderate or high risk category (i.e. T2*<20 ms)
indicating that deferasirox not only chelates the cardiac iron from iron
overloaded myocardium but also prevents further cardiac siderosis by
continuing to remove total body iron [2]. LVEF showed decline of 4.8%
after therapy (P=0.061) but remained well within normal range. In
thalassemia, EF is supranormal in the beginning due to anemia and
hyperdynamic circulation which actually normalizes with transfusion
therapy thereby explaining this apparent paradox. Increase in ESV and
EDV value, which remained within normal range, can also be explained
similarly.
Although the sample size of this study was small,
observational bias was tempered as the radiologists reporting MRI T2*
were blinded to therapy. This study sample size was not adequately
powered to evaluate the relationship between transfusion load and
chelator response. The improvement in the cardiac iron load in our
patients was associated with maintained EF/ESV/EDV within the normal
range; hence we did not observe any significant improve-ment in overall
cardiac function. Similar findings have also been reported by other
researchers [2,3,8,14]. LVEF was maintained at approximately 67% in the
EPIC sub study [19] and improved from 65.1% to 66.8% (P=0.0002)
in one year reported by the ESCALATOR study [16].
Adverse events reported with DFX are generally mild
and include mainly gastrointestinal disturbance and rash but 11-38%
patients may have dose-dependent increase in serum creatinine, and 2%
may have increase in liver transaminases [20]. In the present study,
there were no significant adverse effects even after doses of DFX were
escalated to >30 mg/kg/day. Two patients developed transient
maculopapular rash, 2 developed diarrhea, while 3 developed transient
albuminuria (+2). In all these patients, the problem disappeared when
medications were temporarily stopped and did not recur on restarting
therapy. Creatinine elevation was not seen in any case, although 6
patients (20%) had elevation of ALT twice above normal levels needing
dose reduction. This indicates that DFX is well tolerated by Indian
population.
Overall these initial observations are encouraging;
however, long term multicentric studies with larger patients sample size
will help contribute to better understanding of DFX therapy as an iron
chelator. The goal of thalassemia therapy should be regular transfusion
with optimum iron chelation in proper doses to maintain SF and cardiac
iron level within the normal range. As iron chelation is needed for a
life time, long term safety of any iron chelator is important and needs
constant vigilance. Our results should serve as a benchmark for
evaluating DFX chelation therapy in heavily iron overloaded Indian TM
cases, using serum ferritin, cardiac T2* and safety profile measures for
tailoring continued therapy.
Acknowledgment: Dr Deepak Langde for his inputs
and analysis of statistical data and Mrs Gracy Simond, Sister-Incharge
of Thalassemia Transfusion Centre, Nanavati Hospital, for nursing care
and maintaining data of our patients.
Contributors: RM conceptualized the study and was
responsible for care of the patients, analyzing data and finalizing the
script; JA entered and analyzed data, drafted manuscript; PK and BJ did
radiological reporting of cardiac MRI and T2* and also contributed to
final manuscript.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
• Deferasirox is an effective total body and
myocardial iron chelator.
What This Study Adds?
• Deferasirox is a safe and efficacious iron
chelator in Indian population.
|
References
1. Vichinsky E. Oral iron chelators and treatment of
iron overload in pediatric patients with chronic anemia. Pediatrics.
2008;121:1253-6.
2. Pathare A, Taher A, Daar S. Deferasirox (Exjade®)
significantly improves cardiac T2* in heavily iron-overloaded patients
with b-thalassemia major. Ann Hematol. 2010;89:405-9.
3. Pennell DJ, Berdoukas V, Karagiorga M, Pennell DJ,
Berdoukas V, Karagiorga M, et al. Randomized controlled trial of
deferiprone or deferoxamine in beta-thalassemia major patients with
asymptomatic myocardial siderosis. Blood. 2006;107:3738-44.
4. Tanner MA, Galanello R, Dessi C, Smith G, Westwood
MA, Agus A, et al. A randomized, placebo-controlled, double-blind
trial of the effect of combined therapy with deferoxamine and
deferiprone on myocardial iron in thalassemia major using cardiovascular
magnetic resonance. Circulation. 2007;115: 1876-84.
5. Vichinsky E, Pakbaz Z, Onyekwere O, Porter J,
Swerdlow P, Coates T, et al. Patient reported outcomes of
Deferasirox (Exjade ICL670) versus Deferoxamine in sickle cell disease
patients with transfusional hemosiderosis. Substudy of randomized open
label phase II trial. Acta Hematol. 2008;119:133-41.
6. Taher A, Al Jefri A, Elalfy MS, Al Zir K, Daar S,
Rofail D, et al. Improved treatment satisfaction and convenience
with Deferasirox in iron-overloaded patients with b-thalassemia:
Results from the ESCALATOR trial. Acta Haematol. 2010;123:220-5.
7. Kirk P, Roughton M, Porter J, Walker J, Tanner M,
Patel J, et al. Cardiac T2* magnetic resonance for prediction of
cardiac complications in thalassemia major. Circulation. 2009; 120:
1961-8.
8. Pennell DJ, Porter J, Cappellini M, Beshlawy A,
Chan L, Aydinok Y. Efficacy of deferasirox in reducing and preventing
cardiac iron overload in â-thalassemia. Blood. 2010;115:2364-71.
9. Anderson LJ, Westwood MA, Prescott E, Walker JM,
Pennell DJ, Wonke B. Development of thalassemic iron overload
cardiomyopathy despite low liver iron levels and meticulous compliance
to desferrioxamine. Acta Hematol. 2006;115:106-8.
10. Merchant RH, Joshi A, Ahmed J, Krishnana P,
Jankharia B. Evaluation of cardiac iron load in thalassemia by cardiac
magnetic resonance. Indian Pediatr. 2010 Nov 30 (Epub ahead of print).
11. Tanner MA, Galanello R, Dessi C, Westwood MA,
Smith GC, Nair SV, et al. Myocardial iron loading in patients
with thalassemia major on deferoxamine chelation. J Cardiovasc Magn
Reson. 2006;8;543-7.
12. Wood JC, Kang B, Thompson A, Giardina P, Harmatz
P, Glynos T, et al. The effect of deferasirox on cardiac iron in
thalassemia major: impact of total body iron stores. Blood.
2010;116:537-43.
13. Tanner MA, Galanello R, Dessi C, Smith GC,
Westwood MA, Agus A, et al. Combined chelation therapy in
thalassemia major for treatment of severe myocardial siderosis with left
ventricular dysfunction. J Cardiaovascular Magn Reson. 2008; 10:12.
14. Cappellini MD, Porter JB, El-Beshlawy A, Li Chi
K, Seymour JF, Elalfy M, et al. Tailoring iron chelation by iron
intake and serum ferritin: the prospective EPIC study of deferasirox in
1744 patients with transfusion dependent anemias. Hematologica.
2010;95:557-66.
15. Tahir A, Cappellini MD. Update on the use of
deferasirox in management of iron overload. Therc Clin Risk Manag.
2009:5:857-68.
16. Tahir A, El-Beshlawy A, Elalfy MS, Al Zir K, Daar
S, Damanhouri G, et al. Efficacy and safety of deferasirox, an
oral iron chelator, in heavily iron-overloaded patients with a-thalassaemia:
the ESCALATOR study. Eur J Haematol. 2009;82:458–65.
17. Tanner MA, Porter JB, Westwood MA, Nair SV,
Anderson LJ, Walker JM, et al. Myocardial T2* in patients with
cardiac failure secondary to iron overload. (Abstract). Blood.
2005;106:406.
18. Wood JC, Tyszka M, Carson S, Nelson MD, Coates
TD. Myocardial iron loading in transfusion dependent thalassemia and
sickle cell disease. Blood. 2004:1934-6.
19. Pennell D, Sutcharitchan P, El-Beshlawy A,
Aydinok Y, Taher A, Smith G, et al. Efficacy and safety of
deferasirox (Exjade®) in preventing cardiac iron overload in b-thalassemia
patients with normal baseline cardiac iron: results from the cardiac
substudy of the EPIC trial. Blood. 2008; 112: abstract 3874.
20. Choudhry VP, Naithani R. Current status of iron
overload and chelation with deferasirox. Indian J Pediatr.
2007;74:759-64.
|

