Introduction
Toxic epidermal necrolysis (TEN) is a severe
drug-induced life-threatening disease and characterized by fulminant and
wide-spread blisters responsible for epidermal sloughing(1). It is
associated with high mortality and the majority of the patients die from
complications of infection(1). Supportive therapies and antiseptics are
used in patients with TEN. Different drugs such as cyclophosphamide,
pentoxifylline, thalido-mide, cyclosporine and plasmapheresis have been
reported to be useful in single case observations(1). Recently, a few
cases have been treated with human intravenous immunoglobulin (IVIG)(2-4).
Here, we report a case of TEN treated with IVIG and granulo-cyte
colony-stimulating factor (G-CSF) successfully.
Case report
A 2-year-old boy was admitted to a local hospital for
fever and cough, and diagnosed parapneumonic effusion and
ampicillin-sulbactam was begun. Four days later, he was referred to our
hospital because of persisting fever. On physical examination, the right
hemithorax was dull to percussion, and breath sounds were decreased and
diffuse crackles were heard. Hemoglobin was 7.4 g/dL, white blood cell
count 19400/mm3, platelet count 939000/mm3. Erythrocyte sedimentation
rate was 40 mm/h and C-reactive protein 13.4 mg/dL. The chest x-ray
showed opacity on the right hemithorax, left side displacement of
mediastinum. Exudative pleural fluid was obtained by thoracentesis and
culture was negative. He was hospitalized and managed with teicoplanin
(10 mg/kg i.v., once daily) and chest tube drainage. After two weeks,
amikacin was added to the therapy (15 mg/kg i.v, once daily) because of
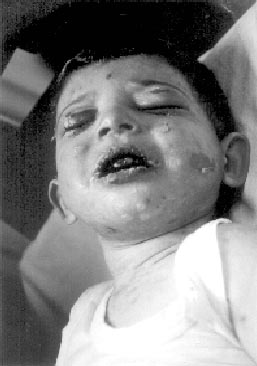
spiking fever. On the 24th day, erythematous rashes developed on his
face and trunk (Fig. 1). It was though as drug reaction and
teicoplanin and amikacin was changed to meropenem (80 mg/kg three times
a day). Hemoglobin was 7.5 g/dL, white blood cell count 6700/mm3;
platelet count 365000/mm3. Liver and kidney function tests were normal.
Antihistaminic was added to the therapy but did not prevent the
occurrence of blisters. Parenteral prednisolone (2 mg/kg/day-totally 30
mg) was started. One day later, his condition worsened, mucocutaneous
detachment and erythema started and effect 90% of the skin surface and
oral and genital mucous membranes. Nikolsky’s sign was positive on the
lesions and normal skin. Arterial blood gas analysis revealed hypox-emia.
The chest x-ray showed bilaterally diffuse pulmonary opacities
suggesting acute respiratory distress syndrome (ARDS). The patient was
placed on an airy bed and benefited from supportive and antiseptic
measures including daily baths. Prednisolone was stopped and IVIG (Sandoglobulin®,
Novartis) was administered twice at a dose of 0.5 g/kg per day (totally
37, 5 g) for 5 consecutive days. A skin biopsy showed full-thickness
epidermal necrolysis and sub-sepidermal blisters with presence of rare
mononuclear cells scattered between necrotic keratinocytes. Candida
albicans was obtained on blood culture and amphotericin B was added to
the therapy. Two days later, white blood cell decreased to 1800/mm3
(absolute neutrophil count 600/mm3) and G-CSF (Neupogen®) was added to
the therapy (5 µg/kg/day, subcutaneously-totally 375 µg) for six
days.
 |
|
Fig. 1. Initial skin involvement;
erythematous rashes and blisters on the face and trunk. |
Two days later, new blisters did not develop and
resolved within 10 days. The cutaneous alterations improved and
erythe-matous rash vanished in two weeks. The re-epithelialization was
completed within one month. The patient recovered satisfactorily.
Discussion
Toxic epidermal necrolysis is character-ized by rash,
bullae and diffuse exfoliation of wide cutaneous surface areas that is
mostly observed secondary to drugs such as co-trimoxazole,
anticonvulsant and nonsteroidal anti-infalmmatory analgestic(5). In our
case, teicoplanin and amikacin were the possible provocative agents.
There was not any proven circulating bacterial toxin in our case, also
the pattern of eruptions did not fit to erythema multiforme, and
epidermal detachment was affecting 90% of the total body area, therefore
we rule out other vesiculobullous diseases. The pathological examination
was also confirming the diagnosis of TEN.
The precise pathomechanisms of TEN remain unknown. It
has been purposed that drugs or their metabolities act as haptens and
render keratinocytes antigenic by binding their surface(6).
Immunohistological studies on cutaneous biopsies of the patients
affected by early stage of TEN have shown a widespread dermal infiltrate
in dermal-epidermal junction, suggesting a cytotoxic cell mediated
reaction versus keratinocytes(6). This cell-mediated immune response
leads to keratinocyte death by apoptosis, involving CD95 (Fas) cell
surface receptor ligand system(6,7). Fas ligand system is normally
expressed on keratinocytes and up regulated by cytokines and skin
infiltrating immune competent cells(8). In TEN, keratinocytes express
large amounts of lytically active Fas ligand that induces apoptosis of
Fas + cells as keratino-cytes(7). IVIG involves the inhibition of Fas
mediated keratinocyte apoptosis by Fas blocking antibodies contained in
IVIG preparation and also it decreases life-threatening infections. We
used IVIG in our patient 24 hours later after the appearance of first
skin lesions. Two days later, epidermal detachment was interrupted and
complete skin re-epithelialization was completed within the two weeks.
We think that poor prognostic factors were delayed the skin re-epithelialization
in our patient.
Neutropenia is regarded as negative prognostic
factor(9). In our case, neutropenia was related with bone marrow
suppression caused by teicoplanin, amphotericin B and sepsis. The
neutrophil count recovered to normal level within six day after G-CSF
administration. Pulmonary involvement is another poor prognostic factor
and occurs in 25% in TEN(10). It may occur as a result of mucosal
sloughing of the tracheobronchial tree.
As a result, our patient had excellent outcome with IVIG and G-CSF
added to basic symptomatic therapy, despite the poor prognostic factors.
Even though, we could not show CD95 receptor disappearance by
immunohistological methods during the therapy, we though that the
successful recovery was related with IVIG, IVIG must be at the top of
the list in infants for the treatment, because of severe and diffuse
mucocutaneous detachment as in our case is a risk factor for sepsis, and
also G-CSF must be added to the therapy when the neutropenia is present.
