Cold chain is the system of transporting, storing and
distributing vaccine in a potent state at recommended temperature till
it is administered to an individual. It is the vital link between the
child and immunity in immunisation against vaccine preventable diseases
(VPD). However potent a vaccine may be, if cold chain is not maintained
from the manufacturer to the place of vaccination, the vaccine efficacy
greatly suffers(1). Most vaccines lose their potency by heat and
sunlight and hence need protection from both(2).
In spite of better development of safe transport and
storage facilities, there are still weak points in the cold chain(3).
The avoidable errors are reported even from developed countries and
include high temperature during storage and transport, exposure of
adsorbed vaccine to freezing temperature, refrigerator without
thermo-meters and lack of regular recordings of temperature,
refrigerator not being used exclusively for vaccine, failure to discard
unused vaccine and use of reconstituted vaccines after exposure at
ambient tempera-ture(4). Each exposure of the vaccine to an ambient
temperature has a cumulative effect on reducing its potency. This is of
concern in view of the fairly frequent reports of VPD occurrence in
populations thought to have been well immunized(5).
There is no simple and cheap method that can be used
in the field to assess whether an exposed vaccine has retained at least
the minimum required potency. The available methods like accelerated
degradation test, viral titers and biological assays are costly and time
consuming taking several months. But the vaccine vial monitor (VVM) now
provided with OPV can indicate the level of heat exposure of that
particular vial. Know-ledge of vaccine’s stability, especially of the
rate of decline in potency at a given temperature can be helpful in
determining its storage requirements.
Vaccine Vial Monitors (VVM)
VVMs, which measure exposure to heat, are time and
temperature sensitive labels attached to vials of vaccine at the time of
manufacture. Through a gradual colour change they warn about the falling
potency. They are designed to meet the vaccine’s heat stability curve as
per WHO or manufacturer requirements(6). VVMs were first introduced on
OPV vials supplied to UNICEF and WHO in 1996.
The information delivered by a VVM is simple. If the
inner square is of lighter colour than the outer reference ring, the
vaccine can be used. If the inner square is of the same colour or darker
than outer ring, the vaccine should not be used (Fig. 1). The
VVMs can be seen as a catalyst for much needed changes in strategies of
vaccine distribution via the cold chain. It will definitely reduce
distribution costs and increase flexibility in handling of vaccines in
the field, thus helping to make operations more effective.
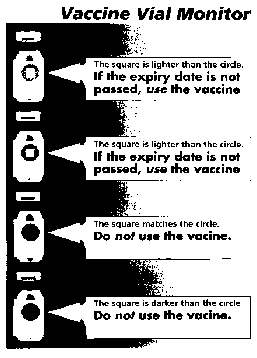
Fig 1. Vaccine vial monitor
Operationalisation of Cold Chain
The cold chain is not simply a series of warehouses
and refrigerated containers, isothermic boxes and portable ice-boxes, it
also involves intermediate phases in which transporters, programme
administrators, warehouse workers and vaccinators have a part to play.
The cold chain involves two complementary aspects: (i) the set
chain represented by the refrigerator, (ii) the mobile chain
represented by isothermic boxes and iceboxes, and (iii) the third
and an important aspect is the personnel in charge of cold chain(5,7).
(a) Walk in cold rooms (WIC): They
are located at regional levels and are meant to store vaccines up to 3
months and they serve 4-5 districts.
(b) Deep freezers and domestic/ice lined
refrigerators (ILR) (300 L): They are supplied to all districts
and WIC locations to store vaccines. They are used for long term
storage of vaccine below –20º C and also for making ice packs. OPV and
measles vaccines can also be stored in deep freezers. The temperature
monitoring is done twice a day with an alcohol thermometer for deep
freezer and a dial thermometer in case of ILR.
(c) Small deep freezers and ILRs (140 L) are
provided to PHCs and clinics.
(d) Cold boxes or isothermic boxes are well
insulated, solid and hermetically (air tight) sealed boxes. They are
supplied to all peripheral centers and used for trans-portation of
large amount of vaccines and to carry them for several days. They are
also useful during electricity failure. Cold accumulators are placed
between the boxes of vaccine and the sides of a box. A paper or
polythene is kept between vaccine and accumulators to prevent freezing
of vaccines because of direct contact.
(e) Vaccine carrier or ice boxes are used to
carry small quantity of vaccines for distribution or to carry the
vaccines to the outreach place of immunization session. It is
surrounded by cold accumulators from inside.
(f ) Day carriers are of the size of a small
lunch box and are used to carry still smaller quantity of vaccines,
but can be used only for a few hours. Here two fully frozen ice packs
are used.
(g) Ice packs should be made of plain water.
Never add salt to the water. Water should be filled in the icepack up
to the level marked. Dry carbon dioxide can also be used instead of
water.
(h) Distribution of vaccines: Only
small quantity of vaccine is distributed to the periphery as break in
cold chain is common at the periphery because of either lack of
knowledge or electricity failure. Vacuum flask is never used for an
outreach place.
Refrigerator
Domestic refrigerator is used for short-term storage
of vaccines. The usual temperature of an ordinary chamber of the
refrigerator is between 4ºC to 10ºC and that of ice chamber or freezer
compartment is between 0ºC to –4ºC. Tips for proper functioning of the
refrigerator are provided below(1,2,5,7).
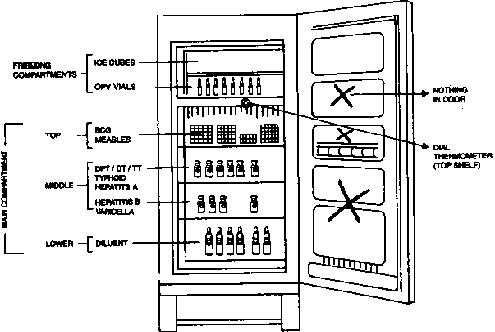
Keep the refrigerator away from sunlight and at
sufficient (10 cm) distance from the wall. Place cold accumulators in
freezer compartment. They can be of use during electricity failure or in
iceboxes. The shelves should be filled with water filled plastic
bottles, which help to maintain temperature during electricity
breakdown. These bottles are not to be used for drinking purpose. The
temperature of the refrigerator is recorded at least twice a day. The
length of electricity breakdown is to be noted and during this period
vaccines are to be protected by putting them in isothermic boxes. When a
layer of ice measuring 5 mm or more forms on the sides of the freezer
compartment, the refrigerator should be defrosted. During defrosting,
the vaccines should be temporarily placed in an isothermic box.
Tips for proper storage
No foods or drinks or other drugs are to be kept in a
refrigerator. It is to be used exclusively for storage of vaccines. To
maintain the temperature, it is opened only when necessary. No vaccine
should be placed in the door panel or in baffle tray (baffle tray may
contain water and this may freeze to ice). Direct contact of vaccine
with ice is to be avoided. An empty space must be left between packages
for free air circulation. Minimum stock of vaccine is to be kept, say
for a month only. Avoid freezing of diluents as the vial may burst when
frozen. Correct storage of vaccines in clinical set up is shown in
Fig. 2.
Thermostability of Vaccines
Knowledge of a vaccine’s stability, especially the
rate of decline in potency at a given temperature, can be helpful in
determining the storage requirements(3). Adsorbed diphtheria and tetanus
toxoids are the most stable of the vaccines commonly used in UIP and OPV
is the most sensitive to heat. Opened vials which have not been fully
utilized after reconstitution should be discarded within one hour, if no
preservative is present (most live virus vaccine) or within 3 hours, or
at the end of the session when the vaccines containing preservative are
used(4). Reconstituting the vaccine with warm diluent may be harmful and
vaccine loses its potency within hours.
Diphtheria and tetanus toxoids
Adsorbed diphtheria and tetanus toxoids in monovalent
form or as components of combined vaccines are the most stable of the
commonly used vaccines. They are stable at elevated temperatures, even
for long periods of storage, but they may change their appearance and
potency when frozen. The freezing of adsorbed vaccines (DPT, DT, TT, HB)
constitutes an absolute contraindication for their use as they are
associated with a reduced immune response or an increased incidence of
local reaction. This is not because of the characteristics of the
toxoids them-selves, but because of an aluminium-based adjuvant, which
changes its structure. The freezing point for adsorbed DTP vaccine is
between –5ºC and –10ºC. The freezing time depends upon the number of
doses in the vial and the temperature. It takes about 110 to 130 minutes
at –10ºC. When vaccine is frozen, the aluminium oxide loses its
colloidal structure and is broken down into crystalline parts, which may
cause aseptic abscesses at the injection site and make the vaccine
ineffective. Frozen adsorbed vaccines contain granular or flaky
particles when thawed. When shaken, they sediment within 30 minutes,
leaving a deposit below a column of transparent fluid. This indicates
that the vaccine has been frozen (shake test)(7,8). The thermostability
is as shown in Table I. Several studies showed an insignificant
decrease in potency when the vaccine was stored for 1.5 years at 18º C,
for 6-12 months at 24º C and for 2-6 months at 37º C(9,10).
Pertussis Vaccine
Thermostability of this vaccine is as described in
the Table I. The effect of freezing the vaccine is same as with
DPT. There are no data available for acellular pertussis vaccine and so
the stability profile similar to that of other protein vaccines is to be
expected i.e., relatively good thermostability, poor resis-tance
to freezing and shelf life of 2-3 years at 2ºC to 8ºC(11).
Table I__Stability of Commonly Used Vaccines(3)
Vaccine
|
|
|
0-8
|
22-25
|
35-37
|
Over 37
|
Tetanus and
diphtheria
toxoids
as monovalent
or component
of combined
vaccines
|
Stable for
|
Stable for Months
|
Stable for
|
At 45ºC stable for
|
3-7 years
|
|
weeks
|
2 weeks. At 53ºC
|
|
|
|
loss of potency
|
|
|
|
after few days. At
|
|
|
|
60-65ºC loss of
|
|
|
|
potency after
|
|
|
|
few hours.
|
Hepatitis B
|
Stable for 2-4
|
Stable for months
|
Stable for weeks
|
At 45ºC Stable
|
|
years
|
|
|
for days
|
Measles
|
Stable for 2
|
Satisfactory
|
Satisfactory
|
At 41ºC: 50%
|
|
years
|
potency up to 50%
|
potency up to 50%
|
loss of potency
|
|
|
for at least 1 month
|
for at least 1 week,
|
after 2-3 days;
|
|
|
|
but may lose 20%
|
at 54ºC: 80%
|
|
|
|
for 1-4 days and
|
loss after 1 day.
|
|
|
|
50% for 2-6 days
|
|
|
|
exposure
|
Pertussis
|
Stable for 18-24
|
Stability varies:
|
Stability varies:
|
At 45ºC:
|
|
months inspite of
|
some vaccines
|
some lose 50%
|
about 10% loss of
|
|
continuous slow
|
stable for 2 weeks
|
during storage for
|
potency per day.
|
|
decrease in
|
|
one week
|
At 50ºC: rapid
|
|
potency
|
|
|
loss in potency.
|
BCG
|
Stable for 1 year
|
Stability varies: 20-
|
Stability varies:
|
Unstable: at
|
|
|
30% loss of
|
20% loss of
|
70ºC 50% loss
|
|
|
viability during 3
|
viability during 3-
|
during 30
|
|
|
month exposure
|
14 day exposure
|
minute exposure
|
OPV
|
Stable for 6-12
|
Some vaccines may
|
Unstable: VVM
|
Very unstable:
|
|
month
|
retain titers for 1-2
|
in use. Loss of
|
At 41ºC 50%
|
|
|
week exposure
|
satisfactory titer in
|
loss in one day.
|
|
|
|
1-3 days.
|
At 50ºC loss of
|
|
|
|
|
satisfactory titer
|
|
|
|
|
after 1-3 hour
|
|
|
|
|
exposure.
|
Rabies HDCV
|
Stable for 3-5
|
Retained
|
Stable for 4 weeks
|
No data available
|
|
years
|
immunogenicity for
|
|
|
up to 11 weeks
|
Live oral
|
Needs
|
Prolonged storage
|
Rapid decrease in
|
No data available
|
tyhpoid Ty21a
|
refrigeration,
|
resulted in
|
viable count.
|
|
shelf life
|
progressively lower
|
Retains minimum
|
|
depends upon
|
viable count
|
potency after 12
|
|
residual moisture
|
|
hours exposure
|
|
content
|
Hepatitis B Vaccine
HB vaccine is a liquid suspension consisting of
purified hepatitis B surface antigen (HBsAg) adsorbed on aluminium salt.
At temperatures of 2ºC to 8ºC, HB vaccine appears to be stable for many
years, but the upper limit of storage life has not been defined (average
being 4 years). Several studies revealed that vaccine heated up to
different temperature for a varied time did not alter reactogenicity and
was equally effective. In one study, at room temperature of 20ºC to
26ºC, vaccines from three manufacturers were found stable for at least
one year. One brand was found to be stable and effective after exposure
at 45ºC for one week and 37ºC for one month(12). It is thus in the upper
range of heat stability, together with tetanus and diphtheria toxoids.
Although HB vaccine is extremely heat stable, there are not yet enough
data to recommend using it entirely outside the cold chain. There is
however scope for developing a management instruction that would allow
removal of the vaccine from the cold chain in emergencies or in outreach
activities of short duration. This vaccine is not to be frozen as with
other adsorbed vaccines. The freezing point for HB vaccine is about
–0.5ºC.
Measles Vaccine
In recent years significant progress has been made in
improving heat stability of measles vaccine because of laid down WHO
criteria as given below and use of effective stabilizers(13). The
criteria are: (i) freeze dried vaccine should retain at least
1000 live virus particles in each dose at the end of incubation at 37ºC
for seven days, and (ii) if during such process titer is
decreased, then it shall have done so by not more than 1 log10.
Freeze dried measles vaccine in lyophilized form is
extremely stable and is not damaged by freezing and refreezing. But it
quickly loses its potency when reconstituted and kept at elevated
temperatures. Thermo-stability of measles vaccine is as shown in the
Table I. The reconstituted measles vaccine remains potent for 24
hours at 4ºC and for 16 to 24 hours at 26ºC(14). But because of
possibility of contamination it is not used for more than 6 hours
i.e. for a single session, at whatever temperature it is stored.
Even during this period it should be protected from elevated temperature
and from light.
BCG Vaccine
BCG was the first vaccine for which a WHO requirement
for heat stability was established. But the standardization of the
stability and studies on it are complicated because of different strains
and manufacturing methods. Accelerated degradation test should be
conducted on each lot of vaccine. Studies have shown that longer storage
of vaccine at elevated temperature reduces post vaccination allergy and
size of vaccination lesions. Most freeze dried BCG vaccines are stable
at temperatures of 0ºC to 8ºC(15).
The stability of the BCG vaccine is affected with
lyophilisation, stabilizer used and proper sealing of vaccine ampoules.
BCG vaccine sealed under vacuum is more stable than one sealed with
nitrogen and argon(15). BCG vaccines in rubber stoppered vials have a
lower stability than those conserved in ampoules(16). BCG vaccine should
be packed in amber glass ampoules to protect it from ultraviolet and
fluorescent light(17).
As with other lyophilized vaccines, BCG vaccine also
should be discarded after 4-6 hours of reconstitution because of risk of
contamination due to lack of preservative and loss of potency.
Oral Polio Virus Vaccine (OPV)
Though OPV vaccine is the least stable vaccine, its
stability has recently been improved with the use of stabilizers like
magnesium chloride. Half-lives of different OPV vaccines when tested in
an Indian study were found to be 4.3 days at 22ºC and 1.7 days at
36ºC(18). OPV supplied by most manufacturers are stable for an extended
period of up to 2 years at –20ºC, for over 6 months at 2ºC to 8ºC and
for over 48 hours at 37ºC. OPV vaccine vial in current use can be stored
at 2º to 8º C in the central compartment of the refrigerator. The
freezing point varies from –6.6ºC to –8.1°C. Most of the times at the
temperature –5ºC of the freezer compartment, OPV may not remain solid.
Repeated thawing and freezing up to 180 cycles within range of –25ºC to
2.5ºC temperature did not show any decline in the titer(19). However,
here the maximum temperature did not exceed 2.1ºC. Under field
conditions, a break in the cold chain can result in vaccines reaching
much higher tempera-tures. Consequently these results are valid only for
situations where the temperature of thawed vaccine remains in the range
found in a refrigerator which is working properly. Repeated thawing of
OPV should be avoided for all practical purposes(l). The WHO
recommendation is that OPV should not be kept at refrigerator
temperatures between 0º C to 8ºC at health centres for more than one
month, nor transported at these temperatures for more than one week(20).
Use ofVVM has made the problem of recognizing the stability of the
vaccine easier.
Thermostability of OPV also depends upon nature of
virus type, nature of stabilizer, pH value of virus suspension,
tightness of stopper and amount of air space above the vaccine.
Mumps and Rubella Vaccine
Thermostability of mumps is similar to that of
measles. The mumps component in Indian MMR shows good stability at 37°C
for up to 21 days. The freeze dried monovalent rubella vaccine and the
rubella component of combined vaccine show low degradation rate. Rubella
is more stable than other components of combined vaccine.
Hepatitis A Vaccine
This is inactivated vaccine and aluminium is used as
an adjuvant. No loss of immunogenicity was observed at 37ºC for up to 3
weeks(21).
Haemophilus influenzae typeB (Hib) Vaccine
PRP-T (Lyophilised) Hib vaccine is stable at
refrigerator temperatures for 36 months and at 25ºC for at least 24
months. After reconstitution it should be discarded within six hours.
Liquid Hib is stable at refrigerator temperature for 24 months.
Typhoid Vaccine
Vi polysaccharide vaccine is highly stable and it
does not require a cold chain even in tropical conditions. This is the
distinct advantage of this vaccine. Immunogenicity of the vaccine is
maintained after storage at 37°C for 6 months and at 22°C for 3
years(22). However it is to be stored in a refrigerator to minimize
degradation.
Live oral typhoid vaccine Ty21a is to be stored at
2ºC to 8ºC. Three lots of vaccine stored at 37ºC for 12 hours maintained
their potency. Evaluation of vaccine after storage for seven days at
20ºC to 25ºC met the potency requirements(23).
Varicella Vaccine
This vaccine is sensitive to light and is readily
inactivated. So it is to be kept away from direct light before and after
reconstitution. Stability is better at –1ºC. It is stable for 2 years at
2ºC to 8ºC. It is to be administered within 30 minutes of
reconstitution(24,25).
Inactivated Poliovirus Vaccine (IPV)
There are differences in heat stability between
various inactivated poliovirus types, with type 1 being most vulnerable.
The stability of the vaccine decreases with thiomersal used as
preservative. The D-antigen content for type 1 drops significantly after
20 days at 24ºC and is undetectable after exposure to 32ºC for the same
period. Type 2 and type 3 are relatively more stable(26). The trivalent
IPV is stable at 2ºC to 4ºC for 1-4 years.
Future Trends
Vaccine distribution without a cold chain would
considerably simplify the delivery system and make it easier to
integrate with drug distribution in developing countries. Sugar-glass
drying technology allows vaccines to be made which can be stored and
transported routinely at tropical room temperatures(27). Extremes of
temperature can be monitored by VVMs.
Long term stabilising ability of trehalose -a
disaccharide is made use of in drying and stabilizing technology in
vaccine manu-facturing. Dried measles vaccine stabilized with trehalose
is found to be stable for two months at room temperature and DTaP for 12
weeks at 60ºC. Only OPV has failed to dry successfully. Though the
results are encouraging, the high cost of regulation and lack of a sure
market have prevented any sugar dried vaccine product from being
licensed.
New multivalent vaccines stabilized with this
technology would be regulated for shelf-life storage at temperate or
tropical room temperatures. Once all vaccines have been stabilized there
will no longer be a need for refrigeration equipment and the associated
maintenance. As a consequence the global savings annually will amount to
approximately $200 million.
Contributors: KRL conceptualized the manuscript
and reviewed the literature. KRL and MKL drafted the paper. MKL will act
as the guarantor for the paper.
Funding: None.
Competiting interest: None stated.
